We have published a more recent review of this organization. See our most recent report on Malaria Consortium's seasonal malaria chemoprevention program.
Malaria Consortium's seasonal malaria chemoprevention program is one of our top-rated charities and we believe that it offers donors an outstanding opportunity to accomplish good with their donations.
More information: What is our evaluation process?
Published: November 2020
Summary
What do they do? Malaria Consortium (malariaconsortium.org) works on preventing, controlling, treating, and eliminating malaria and other communicable diseases in Africa and Asia. We have only reviewed its seasonal malaria chemoprevention (SMC) program, which distributes preventive antimalarial drugs to children 3 to 59 months old in order to prevent illness and death from malaria, typically in four monthly cycles during the high transmission season; our recommendation is just for this part of Malaria Consortium's work. (More)
Does it work? There is strong evidence that SMC substantially reduces cases of malaria. Malaria Consortium has conducted studies in the countries where it has worked to determine whether its programs have reached a large proportion of children targeted. Surveys conducted in 2017-19 found that on average, 93% of targeted children received at least one month of SMC (out of four possible months), 81% received at least two months, 70% received at least three months, and 56% received all four months. (More)
What do you get for your dollar? We estimate that the total cost to achieve the equivalent of four person-months of SMC coverage is $6.59. The numbers of deaths averted and other benefits of SMC are a function of a number of difficult-to-estimate factors, which we discuss below. (More)
Is there room for more funding? We believe that Malaria Consortium could use more funding than it expects to receive to scale up its SMC activities. We estimate that Malaria Consortium could absorb up to $44.7 million for work in 2022-2023. (More)
Malaria Consortium's seasonal malaria chemoprevention program is recommended because:
- SMC is a program with a strong evidence base and strong cost-effectiveness. (More)
- Track record – Malaria Consortium has experience with supporting large-scale SMC programs in seven countries and has demonstrated success at reaching a large portion of targeted children. (More)
- Room for more funding – we believe that Malaria Consortium could productively use more funding than it expects to receive to scale up its SMC activities. (More)
Table of Contents
- Summary
- Our review process
- What do they do?
- Does it work?
- What do you get for your dollar?
- Is there room for more funding?
- Malaria Consortium as an organization
- Sources
Our review process
We began speaking to Malaria Consortium about the possibility of reviewing one of its programs in January 2016.
Over the next several months we tried to determine which of Malaria Consortium's programs we should prioritize evaluating for a possible recommendation, and we ultimately settled on seasonal malaria chemoprevention (SMC). The other programs that we investigated included: bed nets, dengue control, injectable artesunate for severe malaria, integrated community case management (ICCM), micronutrient powders, malnutrition management, neglected tropical diseases morbidity management, integration of nutrition with SMC, prevention of malaria in pregnancy, point of care diagnosis of malaria, and diagnosis of pneumonia.
Our review process has consisted of:
- Extensive conversations with Malaria Consortium staff since 2016.1
- A conversation with a researcher at the London School of Hygiene and Tropical Medicine who has led the work on evaluating the ACCESS-SMC project, a large-scale SMC project led by Malaria Consortium.2
- Reviewing documents that Malaria Consortium shared with us.
What do they do?
Malaria Consortium works on preventing, controlling, treating, and eliminating malaria and other communicable diseases.3 It was established in 2003 and currently works in twelve countries across Africa and Southeast Asia.4 Malaria Consortium's total spending from April 2018 to March 2019 was about $48 million, with about 89% of its spending coming from restricted funds.5
This page focuses exclusively on its seasonal malaria chemoprevention (SMC) programs, which aim to distribute preventive anti-malarial drugs to children 3 months to 59 months old in order to prevent illness and death from malaria.6
The remainder of this section provides more detail on:
- Implementation of SMC programs
- Malaria Consortium-supported SMC programs
- Spending on SMC programs
- Malaria Consortium's role in SMC programs
Implementation of SMC programs
What are SMC programs?
As we wrote in our intervention report on SMC, seasonal malaria chemoprevention is the monthly administration of full preventive courses of antimalarial medicines to children during the malaria season in areas of highly seasonal malaria transmission.7 According to WHO policy recommendation, it "consists of administering a maximum of four treatment courses of SP [sulfadoxine–pyrimethamine] + AQ [amodiaquine] at monthly intervals to children aged 3–59 months in areas of highly seasonal malaria transmission [during the high malaria transmission period]."8 SMC was "formerly known as ‘intermittent preventive treatment of malaria in children [IPTc].'"9
According to the World Health Organization (WHO):
- where more than 60% of the annual incidence of malaria occurs within 4 months;
- where there are measures of disease burden consistent with a high burden of malaria in children (incidence ≥ 10 cases of malaria among every 100 children during the transmission season);
- where SP and AQ retain their antimalarial efficacy.10
According to the WHO, "SMC provides protection for up to [28 days] after each complete (3-day) course…. [Community distributors] should give the dose of SP and the first dose of AQ to the children under their direct observation and should advise the children’s caregivers on how to give the second and third [daily] doses of AQ to the child at home [one dissolved tablet per day, for two days]."11
Malaria Consortium SMC implementation methods
Malaria Consortium supports training of health facility workers and community distributors (CDs) to deliver SMC primarily by going door-to-door.12 Malaria Consortium told us that many CDs are community health workers (CHWs), who are people in the community who work year-round to support basic delivery of health interventions such as vaccines; malaria diagnosis, referral and treatment; nutrition programs, etc. Some CDs are recruited and trained to only deliver SMC and do not provide any other community health services.13 Malaria Consortium told us that CDs are typically paid about $5 to $7 per day for programs such as SMC, though this amount varies by country.14 Malaria Consortium also supports training for supervisors for the program.15
Malaria Consortium typically supports four SMC "cycles." Each cycle includes a four-day distribution period and lasts 28 days, at which point a new cycle starts.16 For each cycle, Malaria Consortium instructs CDs to:17
- determine whether the child is eligible for SMC and give the age appropriate dose.18
- refer all acutely sick children and children with fever to the health facility for evaluation and testing for malaria.19
- directly observe the child swallowing the first dose of dispersible SP+AQ, and then re-dose if the child vomits or spits out all of the medicine within 30 minutes of taking the first dose of the medication.20
- give the child's caregiver 2 tablets of AQ and explain how to give the doses over the following two days.21
- record all doses provided on a tally sheet and mark the wall of the household or compound as visited.22
- advise the child's caregivers to mark a card to record that they've given the other two doses,23 to give the medication again if the child vomits (and to visit the health facility to request replacement doses if this happens), and to take the child to the health facility if they get a fever or are very sick.24
- provide health promotion and malaria prevention messages, including explaining the purpose and benefit of SMC and the importance of children and pregnant women sleeping inside a bed net each night.25
This is a diagram of the delivery schedule:
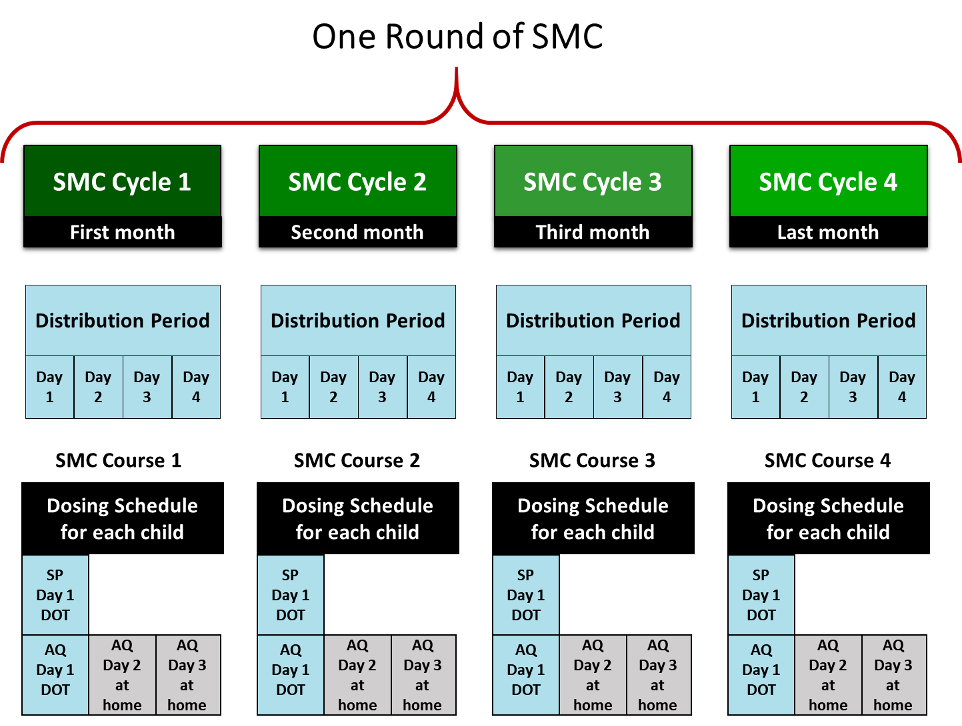
Other relevant aspects of the program are:
- Malaria Consortium instructs CDs not to administer SPAQ to children who: are younger than 3 months or 60 months and older; have a fever or are severely ill (these children should be referred to the local health facility); are taking another sulfa-based medication such as cotrimoxazole; have received another dose of AQ or SP during the past month; or have an allergy to a sulfa-based medication, AQ, or SP.26 Malaria Consortium told us that CDs are trained to follow a checklist on an illustrated job aid, which is translated into local languages,27 in order to check for these issues.28 We do not know how often CDs follow all of the suggested instructions in practice. Malaria Consortium notes that program supervisors oversee CDs to provide mentoring and ensure compliance with SMC guidelines.29
- Malaria Consortium instructs CDs to give a lower dose to children more than 3 but less than 12 months old than they give to children 12 to 59 months old.30 The co-blistered drug packets are color-coded, are different sizes, and include pictures of an infant or a child, and CDs are trained to ask caregivers a set of questions and look for age-related milestones to establish the age of the child.31
- Malaria Consortium supports activities to promote community engagement and social and behavior change before and during SMC campaigns.32
Malaria Consortium also conducts operational research to assess the feasibility and impact of modifying the procedures described above, such as adding a fifth SMC cycle or extending eligibility to children aged 5-10 years. See this spreadsheet for a list of Malaria Consortium's SMC operational research projects in 2019.
More details on how Malaria Consortium assesses the coverage achieved by the SMC programs it supports are below. Malaria Consortium told us that there are several challenges with delivering SMC, including insecurity, flooding, and road access during the rainy season, as well as conflicting community health campaigns, such as vaccination campaigns.33
Malaria Consortium-supported SMC programs
We have seen information from three major SMC projects that Malaria Consortium has supported:
- Pilot and scale-up of SMC in northern Nigeria: The Bill & Melinda Gates Foundation (BMGF) provided about $1.7 million to Malaria Consortium to do operational research on the best way to deliver SMC at scale in Katsina state in northern Nigeria, and then to implement its chosen delivery system and assess its efficiency and impact.34 Malaria Consortium told us that it trained over 3,600 CDs and nearly 200 health workers to provide about 1.6 million courses of SMC to roughly 350,000 children who lived in 4 "local government areas" (LGAs) in northern Nigeria in 2012-2014.35 A major goal of the project was to share what it learned; we have seen a published paper that describes the use of research to inform the design of an SMC intervention in northern Nigeria but have not yet reviewed it in detail.36
- ACCESS-SMC: Unitaid awarded up to $67 million to Malaria Consortium to lead a project called ACCESS-SMC to reach up to 7 million children per year in seven countries in the Sahel region of Africa in 2015-2017.37 The project, which Malaria Consortium described in 2014 as being the "largest-yet global programme" for SMC, concluded in February 2018.38 ACCESS-SMC was led by Malaria Consortium, with Catholic Relief Services as the "primary sub-grantee" and support from many other organizations, including impact evaluation from the London School of Hygiene & Tropical Medicine (LSHTM).39 Malaria Consortium told us that its role in ACCESS-SMC included leading implementation of SMC in three of the seven ACCESS-SMC countries (Burkina Faso, Chad, and Nigeria), overseeing budgets and planning for all ACCESS-SMC activities, and overseeing research (including methodology and presentation).40 Our impression is that ACCESS-SMC paid for almost all aspects of program implementation and monitoring, including medicines and supplies, per diems for CDs, training for CDs, trainers, supervisors, and health facility workers, and research.41
- General SMC program funded primarily by GiveWell-directed funds: Since 2017, Malaria Consortium has been using funding received as a result of GiveWell's recommendation (which we refer to as "GiveWell-directed funds") to support SMC programs in several countries. Over time, the total number of children targeted by these programs has increased from 650,000 in 2017 to 5.8 million in 2019. In 2019, Malaria Consortium used GiveWell-directed funds to target approximately 1.3 million children in 23 districts in Burkina Faso, approximately 1 million children in 20 districts in Chad, and approximately 3.5 million children in 68 LGAs in Nigeria.42 In all three years, GiveWell-directed funds have also supported supervision, monitoring, and evaluation of these programs.43 In 2019, Malaria Consortium also received funding from the Global Fund to Fight AIDS, Tuberculosis, and Malaria to implement SMC in additional states in Nigeria, reaching approximately 340,000 children.44
Malaria Consortium told us that the above programs represent the bulk of Malaria Consortium's work on SMC so far.45
Malaria Consortium's spending on SMC programs
Between January and December 2019, Malaria Consortium spent about $22.2 million in GiveWell-directed funds:46
- $8.1 million (37%) on SMC drugs, along with freight and procurement costs
- $8.5 million (38%) on SMC implementation (including planning for SMC campaigns, training of supervisors and CDs, administration of SMC drugs, community engagement, and monitoring and evaluation, including coverage surveys)
- $2.7 million (12%) on staff and travel costs
- $0.2 million (1%) on research,47 communications, and advocacy48
- $2.6 million (12%) on overhead costs
Of this $22.2 million, Malaria Consortium spent $11 million (50%) in Nigeria, $5.4 million (24%) in Burkina Faso, and $4.4 million (20%) in Chad—or 53%, 26%, and 21% respectively, with above-country spending allocated proportionally across countries.49
For prior work, we have also seen spending data from the pilot and scale-up of SMC in northern Nigeria,50 from 2016 for the ACCESS-SMC program,51 and from 2017 and 2018 for programs supported by GiveWell-directed funds.52
We also provide some information on the estimated cost per child covered of Malaria Consortium-supported SMC programs below.
Malaria Consortium's role in SMC programs
Malaria Consortium's SMC work varies by country, but in general includes the following activities:53
- Determining the quantity of drugs needed, procurement of drugs, and international shipping54
- Procurement and distribution of other SMC commodities55
- Funding distributions, including in-country storage and transportation of drugs and payments to front-line distributors to compensate them for the time they spend on the program
- Technical assistance and logistical support for training SMC implementers, targeted supervision, community engagement and social and behavior change, drug safety, and review of prior implementation and revisions to procedures
- Financial management and oversight, including disbursing funds to local organizations, Ministries of Health, and/or CDs, collecting and validating receipts, and preparing financial reports
- Developing training and supervision materials and training staff at various levels56
- Monitoring and evaluation, including direct observation of program activities by Malaria Consortium staff, funding and coordinating with research firms and academic institutions to conduct coverage surveys and to track changes in malaria incidence and death, and monitoring of drug resistance.
- Advocacy and fundraising with governments, international, multinational, and bilateral organizations, donors, SMC working groups, researchers, and civil society.
- Conducting research that addresses knowledge gaps relating to SMC delivery, quality, and impact, writing and disseminating lessons learned and publications in peer-reviewed journals, and presenting at international global health conferences. See this spreadsheet for a list of Malaria Consortium's SMC operational research projects in 2019.
Malaria Consortium notes that in 2020, it continued to implement SMC during the COVID-19 pandemic, reaching approximately 12 million children. Malaria Consortium established enhanced safety and infection prevention guidance for program delivery, which included providing guidance for SMC delivery by community distributors, procuring items such as face masks and hand sanitizer, and revising training and supervision documents and job aids.57
Does it work?
We base our expectation of the impact of Malaria Consortium's SMC programs on:
- The evidence of effectiveness of SMC at reducing malaria incidence.
- Characteristics of the areas targeted by Malaria Consortium's SMC programs (including malaria incidence and seasonality).
- Evidence that a high proportion of targeted children have received SMC in past rounds.
We believe that the above provides strong indirect evidence that Malaria Consortium's SMC programs reduce malaria incidence in the populations they target. We also consider whether there is direct evidence of such reductions in these populations.
Finally, we consider whether there are factors that are not accounted for in the above evidence that would offset the impact of Malaria Consortium's SMC programs, either through reducing their effectiveness or contributing to negative outcomes.
Is SMC an effective intervention?
Seven randomized controlled trials (RCTs) provide strong evidence that SMC substantially reduces cases of malaria. We discuss this evidence in our intervention report on SMC. We incorporate SMC's impact on malaria incidence, as measured by these RCTs, into our cost-effectiveness model.58
Are Malaria Consortium's SMC programs targeted at areas where they are likely to be effective?
Malaria Consortium told us that before starting work in countries under the ACCESS-SMC program, it conducted an assessment of the overall burden of malaria, transmission and rainfall patterns, regional malaria incidence over time, and seasonal variations in malaria.59 We have not reviewed this evidence in detail, but it seems highly likely to us that Malaria Consortium is working in areas that are suitable for SMC because it is working in countries with high malaria burdens and where it seems that malaria is seasonal.60 More information on our estimates of malaria burden in the countries where Malaria Consortium is working is available in our cost-effectiveness model.61
Are targeted children reached with SMC?
Malaria Consortium conducts coverage surveys to determine what proportion of the target population (children aged 3-59 months) was reached with SMC in the previous round or cycle. We use results from past SMC rounds to understand the impact we should expect future rounds to have. Specifically, we use coverage survey results about the proportion of targeted children reached, along with data on program spending, to estimate the cost of delivering SMC to a child. Our interpretation of these coverage survey results is informed by their comprehensiveness and the methodology used to collect them.
Comprehensiveness
See this spreadsheet for all coverage survey results we have seen from Malaria Consortium's SMC programs. In short, we have seen results from 2015-16 in the seven countries that Malaria Consortium supported through the ACCESS-SMC project and from 2017-19 in the three countries that Malaria Consortium currently supports through its general SMC program (Burkina Faso, Chad, and Nigeria).62 We thus believe that we have seen a thorough picture of the impact of Malaria Consortium's SMC program; we incorporate this assessment into our cost-effectiveness model.63
We focus our review on results from 2017 onward from Burkina Faso, Chad, and Nigeria, as we believe they are more likely to be indicative of what we can expect from future SMC rounds in those countries.
Methodology
Since 2017, Malaria Consortium has conducted two types of coverage surveys. After each of the first three cycles in the SMC round, it conducts a post-cycle coverage survey to measure coverage in the previous cycle only.64 After the fourth and final cycle in the SMC round, it conducts a post-round coverage survey to measure coverage across the full round.65 Both post-cycle and post-round surveys involve household interviews with caregivers of SMC-eligible children. Full details of the methodology used in the surveys we have reviewed are in this spreadsheet.
Below, we summarize Malaria Consortium's general post-cycle and post-round coverage survey methodology and discuss methodological strengths and weaknesses. Overall, we believe that both types of surveys are designed to measure key indicators of the success of SMC campaigns and to achieve samples that are generally representative of target populations. However, we are concerned that the self-reported nature of responses and data collectors' involvement in the later stages of respondent selection may produce bias in results. We are also uncertain about the quality of survey implementation due to the lack of a procedure to audit data collectors' work. We incorporate our assessment of the quality of Malaria Consortium's coverage survey methodology into our cost-effectiveness model66 and into our qualitative assessment of Malaria Consortium's organizational strength.
- Respondent selection: Post-cycle surveys employ lot quality assurance sampling67
in which the program area is subdivided into smaller units, generally of approximately equal population size, and a small number of households is randomly selected from each unit.68
This approach is designed to assess whether each unit met a target coverage level; data from all units can be aggregated to calculate coverage across the program area. Because all units are sampled and are of approximately equal population size, we expect this selection protocol to result in a sample that is generally representative of the target population.69
Post-round surveys employ multi-stage cluster sampling of households in the program area, with sampling units above the household level generally selected randomly with probability proportional to size.70
We expect this selection protocol to result in a sample that is generally representative of the target population.
For both types of surveys, data collectors are instructed to randomly select households and children to survey. In each community, data collectors are instructed to spin a bottle at a central point to choose a direction along which they then select a predetermined number of households at a predetermined interval.71 This method may lead households closer to the center of a community to be overrepresented in the sample. We are unsure of how this might bias results, though it seems plausible that households on the outskirts of a community may have been less likely to be reached by SMC, and thus that results would be biased upward. Next, data collectors enter all eligible children in a selected household, along with whether or not those children received SPAQ in the most recent cycle, into survey software. The survey software then randomly selects two children, independent of whether or not they received SPAQ, from each household. These two children are split into two groups, a "treatment group" and a "non-treatment group." In the non-treatment group, a series of follow-up questions are asked about children who did not receive SPAQ. In the treatment group, no follow-up questions are asked about children who did not receive SPAQ. In both the treatment and non-treatment group, a complete questionnaire is administered for children who did receive SPAQ. Coverage rates are calculated only from the treatment group and are therefore based on a sample in which data were obtained for only one child per household.72
We see data collectors’ involvement in the later stages of respondent selection as a concern. Data collectors may apply selection procedures incorrectly, either unintentionally or intentionally. If, for example, they purposefully select households that are easier to reach, this would be a potential source of upward bias, as households that are easier to reach may also have been more likely to be reached by SMC. We note that we have seen no evidence that data collectors intentionally applied selection procedures incorrectly and note it only as a possibility.
If a selected household is unavailable or refuses to participate in the survey, data collectors are instructed to move to the next household according to the sampling procedure. In the 2019 post-round surveys, 98% or higher of the targeted number of households were interviewed.73 This could mean that non-response rates (i.e. households randomly selected to be interviewed not being interviewed) were low. However, because we do not know how often replacement households were used, it is also possible that a high proportion of interviewed households were replacement households, so these survey completion rates only slightly increase our confidence in the accuracy of results from these surveys. We have not seen survey completion rates from 2019 post-cycle surveys and have not reviewed these rates from prior years.
- Survey design: Malaria Consortium has developed post-cycle and post-round questionnaires,74
which are adapted for use in each country and translated from English into French in Burkina Faso and Chad.75
These questionnaires instruct data collectors to ask caregivers questions about whether their child received SPAQ during the previous cycle (in the case of post-cycle surveys) or during all four cycles of the round (in the case of post-round surveys). Both questionnaires ask about SPAQ provided by a CD on the first day of each cycle and about AQ provided by the caregiver on the second and third days of each cycle. They also ask questions about the quality of program delivery. Data collectors translate questions from English or French into local languages during household interviews,76
which may lead to inconsistencies in translation and reduce the accuracy of results.
A potential source of bias in Malaria Consortium's coverage surveys is their heavy reliance on self-reported responses. Post-round responses are at high risk of recall bias, as they report on up to twelve doses,77 the first of which would have occurred at least three months prior.78 Post-cycle responses are at lower risk of recall bias, as they ask only about three doses and are conducted within a month of those doses. Self-reported responses are also at risk of social desirability bias that could lead caregivers to overreport SMC administration, if they believe that this is the preferred response of data collectors. We expect responses about caregivers' own administration on the second and third days of each cycle to be at greater risk of this type of bias, as they may feel pressure to overreport their own adherence to program guidance. Because the coverage estimates we use are based on responses about CDs' administration on the first day of each cycle,79 about which we believe caregivers may feel less pressure to report positively, this is a smaller concern.
We would have more confidence in a survey that tested the reliability of self-reported responses against some objective measure. In order to verify caregiver responses, data collectors are instructed to review children's SMC record cards and medicine blister packs, if available.80 However, retention of these items has generally been low, leading Malaria Consortium to place low weight on them as indicators of coverage.81
- Survey implementation: Malaria Consortium contracts with local research organizations that recruit data collectors and oversee survey implementation.82
Individuals involved in the surveys generally were not involved in SMC delivery, which suggests that they are unlikely to have a personal interest in survey outcomes.83
Malaria Consortium reports that in Chad, the 2018 post-cycle surveys and the first 2019 post-cycle survey conducted by local research organizations were of low quality.84
This may have impacted the accuracy of results, but we are uncertain about the magnitude or direction of this impact.
Malaria Consortium's coverage surveys do not systematically incorporate an auditing procedure to assess the accuracy of data collectors' work.85 We see this as a methodological weakness, both because such a procedure may encourage accurate data collection and because it would provide a check on the accuracy of results.
- Data capture: Data is collected electronically in both post-cycle and post-round coverage surveys and uploaded to a remote server at the end of each day of data collection.86 One concern we have about coverage surveys in general is that data may be lost after being collected. As mentioned above, for the surveys from which we have reviewed survey completion rates (the 2019 post-round surveys), data was collected and uploaded from 98% or higher of the number of households that were targeted to be interviewed. This leads us to believe that it is unlikely that substantial data loss occurred after collection.
Results
We believe that results from Malaria Consortium's coverage surveys provide strong evidence that a high proportion of the target population was reached with SMC in past rounds. We use these coverage estimates, along with data on program spending, to estimate the cost of delivering SMC to a child.
See this spreadsheet for all results we have seen from Malaria Consortium's SMC programs. Results weighted by target population from post-round surveys in 2017-2019 show that across those years, 93% of targeted children received SMC during at least one monthly cycle, 81% during at least two cycles, 70% during at least three cycles, and 56% during all four cycles.87 In the most recent program year, 2019, post-round surveys measured average coverage across cycles at 89% in Burkina Faso, 69% in Chad, and 68% in Nigeria.88
For coverage surveys in 2017-2019, we have compared results from post-cycle and post-round surveys. After converting the two sets of coverage estimates into a measure of total person-months of coverage for comparison, we find a difference of 2% between them for 2017, 13% for 2018, and 24% for 2019.89 The difference in 2018 is driven largely by results from Nigeria and in 2019 by results from Nigeria and Chad. In all three cases, post-cycle results found higher person-months of coverage than post-round results.90 Differences in sampling protocol and questionnaire design between the two types of surveys may inevitably lead to some difference in results. However, the fact that post-cycle and post-round results were similar in Burkina Faso in 2017-19, in Chad in 2017-18, and in Nigeria in 2017 leads us to believe that larger discrepancies cannot be explained merely by methodological differences and likely result from biases present in the surveys in Chad in 2019 and Nigeria in 2018-19. In all three cases, we believe that the post-round results are a better indication of actual coverage91 and have chosen to use these results to estimate the cost of delivering SMC to a child.
Have malaria rates decreased in targeted populations?
The evidence we have discussed to this point forms the basis of our expectation of the impact of Malaria Consortium's SMC programs. In this section, we consider whether there is additional evidence on the impact or efficacy of SMC that either corroborates this expectation or raises concerns that Malaria Consortium's programs are not achieving the impact we expect.
Sentinel surveillance site data (2013-2016)
We have seen sentinel surveillance site data from Chad, Mali, and Niger for 2013 to 2016 and from Burkina Faso and Nigeria for 2015 to 2016.92 We have received only headline figures for the reduction in malaria cases in children under 5 years old; we have not seen additional information on the sources for or analysis of this data.93 We have therefore not vetted the results (described in the footnote).94 Malaria Consortium told us that the data collected in other ACCESS-SMC countries was of a low quality.
Health Management Information System (HMIS) data
In 2019, Malaria Consortium conducted an assessment of the impact of SMC on malaria rates in Burkina Faso and Chad from 2013 to 2018 and Nigeria from 2017 to 2018, using national HMIS data.95 This assessment found no evidence of impact.96 Malaria Consortium attributes this result to several factors, in particular the variable and generally low quality and completeness of HMIS data used in the analysis.97
Malaria Consortium notes that it chose to analyze HMIS data, despite the fact that it is typically of low quality, because it is an inexpensive and commonly used source of impact data and therefore a relatively sustainable method for assessing impact over the long term.98 Given our understanding that collecting high-quality health facility data is difficult, we find Malaria Consortium's explanation plausible.
Our overall assessment of the expected impact of SMC places little weight on the lack of impact found in HMIS-based impact assessments because:
- Non-randomized comparisons between areas in which SMC is implemented and not implemented may over or understate the impact of SMC due to differences in the two groups other than presence of SMC.
- We believe that there is a risk that data quality limitations will be emphasized more where non-positive trends are found than where positive trends are found. Independently assessing the quality of HMIS data would be a large project for us and we have not prioritized that work.
As noted above, we base our expectation of the impact of Malaria Consortium's SMC programs on malaria rates on the evidence of effectiveness of SMC at reducing malaria incidence, as measured by RCTs, and evidence from Malaria Consortium's coverage surveys that a high proportion of targeted children have received SMC.
Case-control studies
Malaria Consortium also shared case-control studies designed to measure the efficacy of SMC from five ACCESS-SMC countries: Burkina Faso, Chad, The Gambia, Mali, and Nigeria. We have not yet vetted the methodology (some details in the footnote), and the dataset from Nigeria did not pass quality control.99 Malaria Consortium's conclusion from these studies is, "These results confirm that SMC treatments are providing a very high degree of personal protection from malaria for a period of 28 days after each treatment. Protection then declines rapidly emphasizing the importance of repeating treatments at monthly intervals."100 More details on those results in this footnote.101
Northern Nigeria (2012-2014)
We have seen three types of analyses of the impact of Malaria Consortium's northern Nigeria program on malaria indicators. These results seem to be consistent with the impacts of SMC found in randomized controlled trials of the program, but due to our remaining questions about the studies we do not yet see them as strong additional evidence for the impact of SMC programs. See our previous review of Malaria Consortium for more details on this study.
Are there any negative or offsetting impacts?
In this section, we consider factors that are not accounted for in the above evidence that could offset the impact of Malaria Consortium's SMC programs, either through reducing their effectiveness or contributing to negative outcomes.
- Drug resistance: Mass delivery of SMC medicines could contribute to increased drug resistance of SP and/or AQ.102 In 2015, ACCESS-SMC funded a baseline study of the prevalence of gene mutations in malaria parasites that are markers of drug resistance for the drugs used in SMC.103 A follow-up survey was conducted in 2017, following two rounds of SMC.104 We have lightly reviewed a preliminary report from this survey, which reported that between baseline and follow-up, the prevalence of mutations associated with AQ resistance did not increase and that the prevalence of mutations associated with SP resistance did increase.105 It also reported that no samples were collected that contained both mutations associated with AQ resistance and mutations associated with SP resistance.106 We have received the final report from this survey but have not yet reviewed it in depth. In our cost-effectiveness model, we make a small downward adjustment in our estimate of SMC's impact to account for the possibility of development of drug resistance.107
- Possible "rebound" effects: There is a potential concern that SMC could reduce the natural development of immunity to malaria. After children turn five years old and are no longer eligible to receive SMC or if SMC programs are interrupted by lack of funding or other problems, children could lack immunity and be more susceptible to malaria, especially if other prevention methods, such as long-lasting insecticide-treated nets (LLINs), are not used. We have not yet investigated this concern in-depth. In our cost-effectiveness model, we make a small downward adjustment in our estimate of SMC's impact to account for the possibility of rebound effects.108
- Side effects of SMC drugs: Our impression is that the most common reaction seen with SMC drugs in earlier programs was vomiting (AQ is bitter and hard to swallow if not crushed into a powder and mixed with water); more recently, AQ has been available as an orange-flavored dispersible.109 Malaria Consortium told us that the incidence of vomiting has decreased with the new dispersible formulation.110 If the child expels the drugs within 30 minutes, they are supposed to be re-dosed once; we are unsure whether caregivers typically request extra tablets of AQ from CDs when this happens at home to ensure their child gets a full course of SPAQ.111 Our impression is that other side effects from these drugs are rare and include diarrhea, itching, headache, mild abdominal pain, and rash.112 Severe adverse effects associated with SPAQ are rare. A study in Nigeria that followed up with 10,000 SMC recipients one week after receiving SMC resulted in only five reports of severe adverse events, though Malaria Consortium believes there may have been reluctance to report issues.113 Malaria Consortium shared three resources that report very low severe adverse event rates from SMC drugs (available in the following footnote).114 We have lightly reviewed resources on severe adverse event rates from SMC drugs here. In our cost-effectiveness model, we make a small downward adjustment in our estimate of SMC's impact to account for the possibility of severe adverse effects.115
- Drug quality and dosage: Malaria Consortium told us that its policy is to only procure products from suppliers that meet WHO guidelines for pre-qualification quality assurance standards,116 and that these products are approved and quality-assured by the governments of countries where Malaria Consortium works.117 We have not yet asked Malaria Consortium for the details of these processes. If there were issues with drug quality or dosage, it could reduce the effectiveness of the intervention and lead to more rapid development of drug resistance.
What do you get for your dollar?
Cost per child covered
In order to make program costs comparable across all of our top charities, we aim to estimate the total cost to all actors of supporting a given program. Our estimate of cost per child covered for SMC includes research costs, start-up costs, and costs incurred by actors such as governments. Our estimates rely on coverage survey estimates to approximate the number of people reached. With these assumptions, we estimate that the total cost to achieve the equivalent of four person-months of SMC coverage is about $6.59. Full details in this spreadsheet.
We start with this total cost figure and apply adjustments in our cost-effectiveness analysis to account for cases where we believe the charity's funds have caused other actors to shift funds from a less cost-effective use to a more cost-effective use ("leverage") or from a more cost-effective use to a less cost-effective use ("funging").
Cost-effectiveness
SMC programs appear to be in the range of cost-effectiveness of our other priority programs. See our most recent cost-effectiveness model for estimates of the cost per life saved through Malaria Consortium-supported SMC programs and how our model compares this outcome with outcomes of other programs.
Note that our cost-effectiveness analyses are simplified models that do not take into account a number of factors. For example, our model does not include the short-term impact of non-fatal cases of malaria prevented. It also does not include possible offsetting impacts or other harms.118
There are limitations to this kind of cost-effectiveness analysis, and we believe that cost-effectiveness estimates such as these should not be taken literally, due to the significant uncertainty around them. We provide these estimates (a) for comparative purposes and (b) because working on them helps us ensure that we are thinking through as many of the relevant issues as possible.
Is there room for more funding?
We believe that Malaria Consortium could use more funding than it expects to receive to support its SMC work in its current countries of operation (Burkina Faso, Chad, Nigeria, and Togo) in 2022-23. In short:
- Available funding: As of March 2020, Malaria Consortium held $53.5 million in funding available to support its work.
- Expected funding: We project that Malaria Consortium will receive an additional $43 million to support its work over the next three years.
- Spending opportunities: Malaria Consortium has identified opportunities to spend up to $141.2 million over the next three years.
In sum, we estimate that Malaria Consortium could use up to an additional $44.7 million to support its work in its current countries of operation (Burkina Faso, Chad, Nigeria, and Togo) in 2022-23.119
More details and calculations in this spreadsheet. Below, we discuss our approach to assessing Malaria Consortium's room for more funding.
Our approach
In general, we assess top charities' funding needs over a three-year period.120 We ask top charities to report their ideal budgets over the next three years, along with information about their current available funding and funding pipeline. The difference between a charity's three-year budget and the funding we project that it will have available to support that budget is the charity's room for more funding. For this analysis, we focus on the portion of Malaria Consortium's SMC portfolio that is funded entirely or in part by philanthropic funding, including GiveWell-directed funding. We exclude the portion of Malaria Consortium's SMC portfolio that is funded exclusively by institutional funding.121
Available funding
As of March 2020, Malaria Consortium held $53.5 million in uncommitted funding available to support future work in 2021-23. This figure represents the $85 million Malaria Consortium had in the bank,122 less the $31.5 million it had already committed to support its SMC portfolio in 2020.
More details and calculations in this spreadsheet, sheet "Available and expected funding."
Expected funding
We project that Malaria Consortium will receive an additional $43 million to support its work over the next three years. This projection represents our best guess based on past revenue and our understanding of Malaria Consortium's funding pipeline. It excludes any funding we may specifically recommend to Malaria Consortium, beyond the Maximum Impact Fund grant we made in late 2020 and our November 2020 recommendation to Open Philanthropy, both of which are described below. [Note added October 2022: We updated the name of the Maximum Impact Fund in September 2022; more information here.]
We include the following sources of funding in our projection:
- Funding currently held by GiveWell to be granted to Malaria Consortium. We include this amount in our projection of funding available for the next year.
- Funding granted from GiveWell's Maximum Impact Fund. In late 2020, we granted $3.8 million from our Maximum Impact Fund to Malaria Consortium. We include this amount in our projection of funding available for the next year.
- Funding recommended by GiveWell to be granted by Open Philanthropy.123 In November 2020, we recommended that Open Philanthropy grant $27 million to Malaria Consortium to fully support extending its funding runway for its current programs in Burkina Faso, Chad, Nigeria, and Togo through 2022, fully support expanding to newly-eligible locations in Nigeria in 2022 and maintaining work in those new areas in 2023, and partially support extending its funding runway for its current programs through 2023. We include this amount in our projection of funding available for the next year.
- Projected funding due to being a GiveWell top charity. GiveWell maintains a list of all charities that meet our criteria, along with a recommendation for which charity or charities to give to in order to maximize the impact of additional donations. Some donors give based on our top charity list but do not follow our recommendation for marginal funding. We estimate the amount that Malaria Consortium will receive from these donors in the next year and include this amount in our projection of funding available for that year only.124
- Projected unrestricted funding. Our understanding is that Malaria Consortium has not allocated and does not expect to allocate unrestricted funding to its SMC programs (see below). We do not include any unrestricted funding in our projection of funding available to support these programs over the next three years.
More details and calculations in this spreadsheet, sheet "Available and expected funding."
Spending opportunities
Malaria Consortium has identified opportunities to spend up to $141.2 million to support its work in its current countries of operation (Burkina Faso, Chad, Nigeria, and Togo) in 2021-23. This estimate excludes $30.1 million in potential costs of expanding to new countries in 2021-23, which we expect to consider in more detail in the future.125 This estimate is sensitive to Malaria Consortium's best guess of how the COVID-19 pandemic will affect its SMC program costs in the next three years; it is possible that the true impact of the pandemic on these costs will differ from what Malaria Consortium has projected.
After applying Malaria Consortium's available and expected funding, we estimate that Malaria Consortium could use up to an additional $44.7 million in funding over the next three years. Additional funding would support:
- $43.4 million to maintain its SMC programs in Burkina Faso, Chad, Nigeria, and Togo through 2023 at the scale it expects to achieve in 2021.126
- $1.3 million to expand its SMC program in Chad in 2022 and maintain that expansion in 2023.
More details and calculations in this spreadsheet, sheet "Spending opportunities."
Availability of unrestricted funding
Since Malaria Consortium works on a variety of programs, it is possible that receiving additional funds for its SMC work could lead it to reallocate unrestricted funds or other organizational resources (such as time spent fundraising) toward other programs, so that additional dollars donated to Malaria Consortium would not fully support additional SMC work. However, we do not see this as a major concern because we do not believe that Malaria Consortium has substantial unrestricted funding available, and it seems that Malaria Consortium has not allocated substantial unrestricted funding to SMC work in the past (see footnote for details).127
Global funding landscape
Malaria Consortium's estimate of its room for more funding for SMC makes certain assumptions about what funding will be available globally for SMC from other major institutional funders. Historically, major funders of SMC have included the Global Fund to Fight AIDS, Tuberculosis, and Malaria, PMI, the UK government's Department for International Development, and the World Bank.128 Malaria Consortium expects the Global Fund and PMI to continue funding SMC in 2021-23 in the countries where it works (the Global Fund in Burkina Faso, Chad, Nigeria, and Togo; PMI in Burkina Faso and Nigeria). Our understanding is that the World Bank will not fund SMC in these countries in the future. We are unsure how much funding for SMC will be available from the UK government.129
Malaria Consortium shared the information it had, as of June 2020, about the expected size of funding gaps in 2020 in each of the countries that contain areas eligible for SMC. In short:130
- A total of 31 million children are eligible for SMC. This estimate includes target population estimates from twelve countries.131 Malaria Consortium did not have target population estimates from two countries.132
- In the twelve countries, Malaria Consortium estimates that 895,000 eligible children will not be covered in 2020, roughly equivalent to a funding gap of $4.4 million in 2020.133
- This gap is substantially lower than the 9.1 million eligible children that Malaria Consortium estimates were not covered in 2019.134 This is due almost entirely to increased coverage in Nigeria. In 2019, Nigeria was by far the largest part of the global funding gap, with 8 million out of 11.7 million eligible children not covered with SMC.135 Malaria Consortium estimates that the gap in Nigeria has been reduced to 750,000 in 2020, as a result of increased funding from the Global Fund and PMI and increased GiveWell-directed funding.136
Currently, a substantial portion of global funding available for SMC is GiveWell-directed funding of Malaria Consortium's work. We roughly estimate that in 2020, GiveWell-directed funding will constitute 40% of total SMC funding in Burkina Faso, 50% in Chad, and 30% in Nigeria.137
2020 is the last year of the current Global Fund three-year funding cycle; Malaria Consortium's estimate of its room for more funding is informed by the information it had available, as of June 2020, about how the Global Fund will allocate funding over the period 2021-23. At a high level, Malaria Consortium expects funding available from the Global Fund for SMC to increase relative to the previous three-year period, at least in the countries where Malaria Consortium works.138 This suggests that the proportion of eligible children who are not covered, the majority of whom live in Nigeria, will continue to decrease. On the other hand, revised SMC eligibility criteria may lead to the adoption of a fifth SMC cycle in some areas that implement SMC, expansion of eligibility to new areas within countries that implement SMC, and adoption of SMC by countries that do not currently implement SMC.139 Such changes could increase both the total target population eligible for SMC and the average cost per targeted child, thereby increasing the global room for more funding for SMC.
Malaria Consortium as an organization
We use qualitative assessments of our top charities to inform our funding recommendations. See this page for more information about this process and for our qualitative assessment of Malaria Consortium as an organization.
Sources
- 1
- We had one conversation with Malaria Consortium staff in each of January, February, and March, 2016.
- GiveWell's non-verbatim summary of a conversation with Malaria Consortium Staff, August 25, 2016.
- GiveWell's non-verbatim summary of conversations with Malaria Consortium staff, November 7 and November 9, 2016.
- GiveWell's non-verbatim summary of a conversation with Malaria Consortium Staff, November 23, 2016.
- @GiveWell's non-verbatim summary of a conversation with Malaria Consortium Staff, January 18, 2017@.
- GiveWell's non-verbatim summary of a conversation with Malaria Consortium staff, January 19, 2017.
- GiveWell's non-verbatim summary of a conversation with Malaria Consortium staff, March 24, 2017.
- GiveWell's non-verbatim summary of a conversation with Diego Moroso, April 25, 2017.
- Starting in 2017, we deprioritized publishing notes from our conversations with Malaria Consortium. We continued speaking regularly with Malaria Consortium staff in 2017-2018.
- 2
- 3
"Established in 2003, Malaria Consortium is one of the world’s leading non-profit organisations specialising in the prevention, control and treatment of malaria and other communicable diseases among vulnerable populations.
Our mission is to improve lives in Africa and Asia through sustainable, evidence-based programmes that combat targeted diseases and promote child and maternal health." Malaria Consortium website, "Who We Are".
- 4
- "The number of countries we work in will fluctuate year-on-year. Currently it’s 12, including Togo." Malaria Consortium, comments on a draft of this page, October 2020.
- "Established in 2003, Malaria Consortium is one of the world’s leading non-profit organisations specialising in the prevention, control and treatment of malaria and other communicable diseases among vulnerable populations.
Our mission is to improve lives in Africa and Asia through sustainable, evidence-based programmes that combat targeted diseases and promote child and maternal health." Malaria Consortium website, "Who We Are".
- 5
- Malaria Consortium spent 36,440,000 British pounds from April 1, 2018 to March 31, 2019. See Malaria Consortium, 2019 trustees' report and financial statements, "Total expenditure" under column "Group 2019 Total Funds", Pg 18.
- We used X-RATES to find the GBP to USD conversion rate as of March 31, 2019: 1.304. 36,440,000 x 1.304 = 47,517,760.
- Percentage of spending coming from restricted funds calculation: (10,147,000 + 22,143,000) / 36,440,000 British pounds = 0.89. See Malaria Consortium, 2019 trustees' report and financial statements, Pg 18, and compare "Restricted funds" and "Total funds" columns for the "Total expenditure" from "Group 2019."
- 6
"In March 2012, the World Health Organisation (WHO) issued a policy recommendation for a new intervention against Plasmodium falciparum malaria - seasonal malaria chemoprevention (SMC), previously referred to as intermittent preventive treatment in children (IPTc), in children under five years old. SMC is defined as the intermittent administration of full treatment courses of an anti-malarial treatment combination during the malaria season to prevent illness and death from the disease.
The objective is to maintain therapeutic anti-malarial drug concentrations in the blood throughout the period of greatest risk. This will reduce the incidence of both simple and severe malaria disease and the associated anaemia and result in healthier, stronger children able to develop and grow without the interruption of disease episodes. SMC has been shown to be effective, cost effective and feasible for the prevention of malaria among children in areas where the malaria transmission season is no longer than four months." Malaria Consortium website, "Seasonal Malaria Chemoprevention".
- 7
"Across the Sahel sub-region, most childhood malarial disease and deaths occur during the rainy season, which is generally short (3-4 months). Giving effective antimalarial treatment at monthly intervals during this period has been shown to be 75% protective against uncomplicated and severe malaria in children under 5 years of age." World Health Organization, "Seasonal Malaria Chemoprevention".
- 8
World Health Organization, "Seasonal Malaria Chemoprevention: A Field Guide", Pg 7.
- 9
World Health Organization, "Seasonal Malaria Chemoprevention: A Field Guide", Pg 7.
- 10
World Health Organization, "Seasonal Malaria Chemoprevention: A Field Guide", Pg 8.
- 11
World Health Organization, "Seasonal Malaria Chemoprevention: A Field Guide", Pg 9. Malaria Consortium provided the edit to the quotation in response to drafts of this page in October 2017 and in October 2020.
- 12
- "How is SMC delivered?
Local announcements each month will inform the community about the date of SMC, which will be delivered by community health workers at pre-arranged locations in the community, or by visiting each household. Health workers will receive appropriate training before the intervention begins and will be supervised by nurses and the district health team."ACCESS-SMC project brochure, Pg 3. Malaria Consortium noted that health workers are also sometimes trained for SMC programs (comment provided in response to a draft of this page in October 2017). - For Malaria Consortium's SMC program in Nigeria: "House to house delivery method was the most used approach, as reported by 88.2 percent of the respondents. This was similar across the LGAs [local government areas] though Maiadua had slightly higher numbers receiving through the fixed point delivery approach. The duration spent in receipt of drugs in the home was 20 minutes, half the time spent in receipt of drugs from a fixed point which was 47 minutes. Knowledge of the different types of SMC drugs and dose duration was high at over 80 percent. This highlights house to house delivery of SMC as a quicker and most preferred delivery mechanism by the caregivers. There is need for costing the two delivery mechanisms to assess if home based delivery still remains a cost effective delivery channel." Malaria Consortium, Nigeria SMC evaluation report, 2014, Pg 38.
- For ACCESS-SMC delivery methods, see the annexes on Pgs 31-70 of ACCESS-SMC multi-country cost analysis, January 2017. Sample quote: "A combination of 6,500 trained community distributors and 355 health facility staff (e.g. nurses and midwives) administered SMC by way of two distribution methods: door-to-door (two-person teams) and at fixed points located at health centers (one-person teams) which were in place to serve primarily as referral centers for sick children and provide SMC to children who were came to the facility. It was estimated that 90% of SMC was distributed by door-to-door teams and 10% was distributed at fixed points. To ensure the acceptability of SMC and high rates of coverage within communities, 3,483 trained community mobilizers sensitized communities on the benefits of SMC prior to and during each distribution cycle." ACCESS-SMC multi-country cost analysis, January 2017, Pg 31.
- "[Delivery from pre-arranged locations in the community] was for ACCESS only. Now is mostly [household to household]." Malaria Consortium, comments on a draft of this page, October 2019.
- "How is SMC delivered?
- 13
- GiveWell's non-verbatim summary of conversations with Malaria Consortium staff, November 7 and November 9, 2016.
- In response to this review, Malaria Consortium noted that “In most cases this is true. However, due to the number of CDDs/CHWs required to implement SMC we have had to recruit and train other community volunteers who only provide SMC services.” Malaria Consortium emails (unpublished), November 23, 2016.
- Malaria Consortium, comments on a draft of this page, October 2019.
- 14
Malaria Consortium emails (unpublished), November 23, 2016.
Comments from Malaria Consortium in response to a draft of this page in October 2017. - 15
"This includes coordinating the supervision process during SMC monthly delivery to ensure quality of care by providing supervision tools and supervising the supervisors." Malaria Consortium, comments on a draft of this page, October 2019.
- 16
- See Figure 1, Pg 8, Malaria Consortium, "Seasonal Malaria Chemoprevention Programme Start-Up Guide, Nigeria"
- "All three countries are now delivering SMC over 4 days. Previously in ACCESS SMC some countries implemented over 5 days." Malaria Consortium, comments on a draft of this page, October 2020.
- 17
See Malaria Consortium, 2018 SMC Coverage Report, Table 1, Pg. 8 for an overview of SMC procedures.
- 18
Malaria Consortium emails (unpublished), November 23, 2016.
- 19
Comments from Malaria Consortium in response to a draft of this page in October 2017
- 20
- See Figure 1, Pg 8, Malaria Consortium, "Seasonal Malaria Chemoprevention Programme Start-Up Guide, Nigeria". "DOT" stands for "Directly Observed Treatment".
- "Day 1 SP and AQ should be administered by the drug distributor as DOT.
If the child vomits or spits out the drugs within 30 minutes, a second dose should be given." Table 1, Pg. 8, Malaria Consortium, 2018 SMC Coverage Report. - Malaria Consortium, comments on a draft of this page, October 2019.
- 21
- See Figure 1, Pg 8, Malaria Consortium, "Seasonal Malaria Chemoprevention Programme Start-Up Guide, Nigeria". "DOT" stands for "Directly Observed Treatment".
- "How long should the Role Model Caregiver observe each child after giving SMC medicines? a) 10 minutes, b) 15 minutes, c) 30 minutes, d) 1 hour, [Correct answer: C]" Malaria Consortium Quiz Answer Key.
- "What should the Role Model Caregiver advise the child’s caregiver after giving the first dose of the SMC medicines? a) When to take the second and third dose of amodiaquine (AQ) at home, b) The importance of adherence to giving the two doses of amodiaquine (AQ) home, c) What to do if the child vomits, d) How to mark the SMC Record Card after giving each dose and to bring the card back for the next SMC cycle, e) When to go to the health facility if the child gets a fever or very sick, f) All of above, [Correct Answer: F.]" Malaria Consortium Quiz Answer Key.
- "The child’s SMC Record Card is very important because: a) It shows the Role Model Caregiver the name and register number of the child, b) The child’s caregiver should always take it with them if they need to go to the health facility, c) It shows how many times the child received the SMC medicines each month, d) It is made of thick paper and is in a plastic packet, e) a, b and c, f) All of the above, [Correct answer: E.]" Malaria Consortium Quiz Answer Key.
- 22
Malaria Consortium, comments on a draft of this page, October 2019.
- 23
- "The child’s SMC Record Card is very important because: a) It shows the Role Model Caregiver the name and register number of the child, b) The child’s caregiver should always take it with them if they need to go to the health facility, c) It shows how many times the child received the SMC medicines each month, d) It is made of thick paper and is in a plastic packet, e) a, b and c, f) All of the above, [Correct answer: E.]" Malaria Consortium Quiz Answer Key.
- We have seen a few versions of templates for "SMC Record Cards." The latest version that we have seen (from 2016) is here: SMC Record Card Template 2016.
- 24
- See Figure 1, Pg 8, Malaria Consortium, "Seasonal Malaria Chemoprevention Programme Start-Up Guide, Nigeria". "DOT" stands for "Directly Observed Treatment".
- "How long should the Role Model Caregiver observe each child after giving SMC medicines? a) 10 minutes, b) 15 minutes, c) 30 minutes, d) 1 hour, [Correct answer: C]" Malaria Consortium Quiz Answer Key.
- "What should the Role Model Caregiver advise the child’s caregiver after giving the first dose of the SMC medicines? a) When to take the second and third dose of Amodiaquine (AQ) at home, b) The importance of adherence to giving the two doses of Amodiaquine (AQ) home, c) What to do if the child vomits, d) How to mark the SMC Record Card after giving each dose and to bring the card back for the next SMC cycle, e) When to go to the health facility if the child gets a fever or very sick, f) All of above, [Correct Answer: F.]" Malaria Consortium Quiz Answer Key.
- Malaria Consortium told us that caregivers are able to visit CDs to request replacement doses if their child vomits. GiveWell's non-verbatim summary of conversations with Malaria Consortium staff, November 7 and November 9, 2016.
- 25
Malaria Consortium, comments on drafts of this page, October 2019 and October 2020.
- 26
- "Who should NOT get SMC medicines? a) Any child with a fever or who is severely ill, b) Any child who is currently taking a sulfa medication such as co-trimoxazole (Septrin, or Bactrim), c) A child who has received a dose of either Amodiaquine (AQ) and sulfadoxine / pyrimethamine (SP) during the past month, d) A child who is allergic to sulfa medication such as co-trimoxazole, Septrin, or Bactrim, e) A child who is allergic to either Amodiaquine (AQ) and sulfadoxine / pyrimethamine (SP), f) a,b,ande, g) All of the above. [Correct answer is G.]" Malaria Consortium Quiz Answer Key.
- "What important questions must the Role Model Caregiver ask about each child before giving SMC medicines? a) The child’s age, b) If the child has taken any medicines in the past 28 days, and which ones, c) If the child has any allergies, d) If the child has a fever or is sick, e) a, b, and d, f) All of the above, [Correct answer is F.]" Malaria Consortium Quiz Answer Key.
- "What should the Role Model Caregiver do if a child has a fever on the day SMC medicines are being given? a) Complete the SMC Referral Form, b) Refer the child to the nearest health facility for a malaria test, c) Complete the SMC Register with the reason for the referral, d) Give the child Coartem, e) Give the child the SMC medicines to treat the fever, f) a, b, and c, g) All of the above, [Correct answer is F.]" Malaria Consortium Quiz Answer Key.
- 27
"The principle here is that we produce those materials in the most appropriate language. In some cases (for example Hausa in Nigeria), that’s a local language, in other cases (like French in Burkina Faso), it’s the official language." Malaria Consortium, comments on a draft of this page, October 2020.
- 28
Malaria Consortium emails (unpublished), November 23, 2016.
Comments from Malaria Consortium in response to a draft of this page in October 2017. - 29
Malaria Consortium, comments on drafts of this page, October 2019 and October 2020.
- 30
- "SMC medicines come in two colour packets for different age children. What age group should get the medicines in the YELLOW packets? ... [correct answer:] b) 3 to 12 months " Malaria Consortium Quiz Answer Key.
- "SMC medicines come in two colour packets for different age children. What age group should get the medicines in the BLUE packets? ... [correct answer:] b) 12 to 59 months" Malaria Consortium Quiz Answer Key.
- 31
"Determining a child’s age
1. Ask the caregiver how old the child is.
2. Ask to see the child’s vaccination card.
3. If the caregiver does not know the child’s age or does not have a vaccination card; ask the caregiver to describe the events when the child was born. (Dry or rainy season, religious celebrations such as Eid or Ramadan, political, or social events).
4. Look for 1 or more of the following milestones for appropriate age category:
Most infants younger than 3 months will not be able to:
– Hold their head and neck steady when being held upright
– Push down with their legs when their feet are on a hard surface
– Grab an object in their hand and bring it to their mouth
Most children 1 year or older should be able to:
– Sit without help
– Pull themselves up to standing using a chair or caregiver’s hand
– Stand on their own or take a few steps
Most children who are older than 5 years should be able to:
– Raise their arm over their head to touch their opposite ear
– Stand on one foot for 10 seconds or longer
– Hop on one foot"
From unpublished source, "Field Guide for Training and Service Delivery of Seasonal Malaria Chemoprevention – Nigeria." - 32
"The former includes supporting the selection of community distributors by community leaders according to suggested criteria, sensitization/coordination meeting with traditional and religious leaders, training and involvement of lead mothers in the campaign in Nigeria. The latter includes producing radio and TV spots advertising the campaign, as well as training 'town criers' who announce the passing of the campaign." Malaria Consortium, comments on a draft of this page, October 2019.
- 33
Malaria Consortium, comments on a draft of this page, October 2019.
- 34
- "Budget: 1,694,339.00 (USD)" Malaria Consortium, "Support Scale up of Seasonal Malaria Chemoprevention (SMC)". We also searched the Gates Foundation's grant database to see whether it made any additional grants to Malaria Consortium for SMC work, but only saw this grant (grant page available at Gates Foundation, "Malaria Consortium").
- "Malaria Consortium is implementing and assessing the feasibility of a community-based seasonal malaria chemoprevention (SMC) project in Katsina state, northern Nigeria, with funding from the Bill & Melinda Gates Foundation. Following new World Health Organisation policy recommendations on SMC, this project administers full antimalarial treatments during the malaria season in areas with highly seasonal malaria transmission, to prevent illness among children under five." Malaria Consortium, Project Brief: Seasonal malaria chemoprevention, Katsina, Pg 1.
- "The project’s objectives are:
- To design, in consultation with key local stakeholders, an appropriate community-based delivery system for SMC in Katsina state based on formative research, which will review aspects relating to feasibility, community acceptability, effectiveness and cost
- To launch and execute SMC delivery according to the selected delivery system and collect data on process indicators and costs
- To evaluate community acceptability, costs and effectiveness of the delivery system for SMC
- To inform future national and state plans for SMC continuation/ scale up by disseminating findings and sharing experiences with key stakeholders" Malaria Consortium, Project Brief: Seasonal malaria chemoprevention, Katsina, Pg 2.
- It appears that this project may have also been related to another major (£89 million) project that Malaria Consortium was working on in Nigeria called "Support to National Malaria Programme (SuNMaP)".
- "Support to National Malaria Programme (SuNMaP) is an £89 million UK aid funded project that works with the government and people of Nigeria to strengthen the national effort to control malaria. The programme began in April 2008 and [ended] in March 2016.
Led by Malaria Consortium, SuNMaP was jointly managed by a consortium, including lead partners Health Partners International and GRID Consulting, with nine other implementing partners. SuNMaP was implemented in 10 states across Nigeria, including Anambra, Kano, Niger, Katsina, Ogun, Lagos, Jigawa, Enugu, Kaduna and Yobe.
SuNMaP worked with the Nigerian government's National Malaria Elimination Programme (NMEP) to harmonise donor efforts and funding agencies around national policies and plans for malaria control. Project targets were aligned with the National Malaria Strategic Plan and Global Malaria Action Plan. The project aimed to improve national, state and local government level capacity for the prevention and treatment of malaria." Malaria Consortium, SuNMaP Final Report, Pg 38.
- "July 2013: Result of SuNMaP study on efficacy of sulphadoxine‐pyrimethamine (SP) for intermittent treatment against malaria in pregnancy published. SuNMaP commences seasonal malaria chemoprevention in Katsina State." Malaria Consortium, SuNMaP Final Report, Pg 16.
- "Support to National Malaria Programme (SuNMaP) is an £89 million UK aid funded project that works with the government and people of Nigeria to strengthen the national effort to control malaria. The programme began in April 2008 and [ended] in March 2016.
- 35
- Malaria Consortium emails (unpublished), November 23, 2016.
- "Length of project: 2012-2014 (33 months)," Malaria Consortium, Project Brief: Seasonal malaria chemoprevention, Katsina, Pg 1.
- "[In 2013], Total number of children covered = 487,354"; "[In 2014], Total number of children covered = 1,112,330," Cost analysis of the seasonal malaria chemoprevention project in Katsina state, Nigeria, Pg 20. However, compare to "A total of 487,353 treatment courses were delivered in two LGAs over three treatment cycles in the first round of SMC representing an average of 115% coverage over the three cycles. In 2014, a total of 1,078,440 treatments were provided across four LGAs over four cycles with an average of 115% administrative coverage." Malaria Consortium, Monitoring and evaluation summary Nigeria, Pg 2. Our understanding is that about 4 courses of SMC were aimed to be delivered per child, suggesting that about 350,000 to 400,000 children were targeted. (487,354 + 1,112,330) / 4 = 399,921, while (487,353 + 1,078,440) / 4 = 391,448.25.
- "The project also has a number of critical milestones:...85 percent of children targeted receive all courses of SMC in the second round," Malaria Consortium, Project Brief: Seasonal malaria chemoprevention, Katsina, Pg 2.
- [Under "Process of implementation 2"]: "Selection and training of 2,500 community caregivers (CHWs) and supervisors to deliver the intervention and complete the necessary forms," Slide 13, Malaria Consortium, SMC presentation, May 6, 2014. Note: Malaria Consortium gave us an updated figure of 3,600 CDs, which is cited above.
- "Intervention area 2013:
- In consultation with the State MOH and SMCP, four LGAs were chosen
- Two for implementation of SMC and two for control in 2013
- Full implementation in four LGAs in 2014," Slide 9, Malaria Consortium, SMC presentation, May 6, 2014.
- 36
- "The project’s objectives are: ...To inform future national and state plans for SMC continuation/scale up by disseminating findings and sharing experiences with key stakeholders," Malaria Consortium, Project Brief: Seasonal malaria chemoprevention, Katsina, Pg 1.
- Strachan et al. 2016 Abstract:
- "Background: Experience of seasonal malaria chemoprevention (SMC) is growing in the Sahel sub-region of Africa, though there remains insufficient evidence to recommend a standard deployment strategy. In 2012, a project was initiated in Katsina state, northern Nigeria, to design an appropriate and effective community-based delivery approach for SMC, in consultation with local stakeholders. Formative research (FR) was conducted locally to explore the potential feasibility and acceptability of SMC and to highlight information gaps and practical considerations to inform the intervention design.
- Methods: The FR adopted qualitative methods; 36 in-depth interviews and 18 focus group discussions were conducted across 13 target groups active across the health system and within the community. Analysis followed the ‘framework’ approach. The process for incorporating the FR results into the project design was iterative which was initiated by a week-long ‘intervention design’ workshop with relevant stakeholders.
- Results: The FR highlighted both supportive and hindering factors to be considered in the intervention design. Malaria control was identified as a community priority, the community health workers were a trusted resource and the local leadership exerted strong influence over household decisions. However, there were perceived challenges with quality of care at both community and health facility levels, referral linkage and supportive supervision were weak, literacy levels lower than anticipated and there was the potential for suspicion of ‘outside’ interventions. There was broad consensus across target groups that community-based SMC drug delivery would better enable a high coverage of beneficiaries and potentially garner wider community support. A mixed approach was recommended, including both community fixed-point and household-to-household SMC delivery. The FR findings were used to inform the overall distribution strategy, mechanisms for integration into the health system, capacity building and training approaches, supportive interventions to strengthen the health system, and the social mobilization strategy.
- Conclusions: Formative research played a valuable role in exploring local socio-cultural contexts and health system realities. Both opportunities and challenges for the introduction of SMC delivery were highlighted, which were appropriately considered in the design of the project."
- 37
- "UNITAID has awarded up to $67 million to Malaria Consortium to oversee the largest-yet global programme to increase seasonal malaria chemoprevention (SMC) across the Sahel region of Africa, where malaria remains the leading cause of severe illness and mortality in young children...This grant will help increase capacity and reduce prices for SMC products in the seven target countries and is expected to supply an estimated 30 million treatments every year to protect 7.5 million children, preventing around 50,000 deaths." Malaria Consortium, ACCESS-SMC announcement, May 8, 2014.
- "ACCESS-SMC is a UNITAID-funded project, led by Malaria Consortium in partnership with Catholic Relief Services, which is scaling up access to seasonal malaria chemoprevention (SMC) across the Sahel to save children’s lives. This three year project is supported by London School of Hygiene & Tropical Medicine, Centre de Support de Santé International, Management Sciences for Health, Medicines for Malaria Venture, and Speak Up Africa. It will provide up to 30 million SMC treatments annually to 10 million children less than five years of age [specifically, children ages 3 to 59 months] in Burkina Faso, Chad, Guinea, Mali, Niger, Nigeria and The Gambia, potentially averting 49,000 deaths due to malaria." Malaria Consortium, ACCESS-SMC page.
- "ACCESS-SMC is working with all seven supported National Malaria Control Programs (NMCPs) to create SMC delivery pathways. We are providing support in a range of areas, including planning and management, supply chain, health worker training and communications and social mobilization. The project is also procuring almost 15m doses of SMC drugs in 2015, and up to 30m in 2016. Working together with NMCPs, we will reach 3.3m children in 2015 and up to 6.6m children by 2016 in Burkina Faso, Chad, Guinea, Mali, Niger, Nigeria and The Gambia." ACCESS-SMC website, "The Project".
- Clarifications around number of children targeted annually from Malaria Consortium emails (unpublished), November 23, 2016.
- "Malaria Consortium implemented SMC with UNITAID funding under the ACCESS-SMC project between 2015 and 2017 (three SMC rounds), with an original geographical scope encompassing 7 countries (Burkina Faso, Chad, Guinea, Mali, Niger, Nigeria and The Gambia). In 2017, the project focused on just three countries, Burkina Faso, Nigeria and Chad, where both Malaria Consortium and other stakeholders had more difficulties identifying alternative sources of funding (either domestic of international from sources such as the Global Fund, PMI or the World Bank) to transition out of ACCESS-SMC." Malaria Consortium, Summary Update, May 2018, Pg. 1.
- 38
- "UNITAID has awarded up to $67 million to Malaria Consortium to oversee the largest-yet global programme to increase seasonal malaria chemoprevention (SMC) across the Sahel region of Africa, where malaria remains the leading cause of severe illness and mortality in young children." Malaria Consortium, ACCESS-SMC announcement, May 8, 2014.
- "The original ACCESS-SMC grant was expected to end on August 31st, but Malaria Consortium secured a cost extension up to February 28, 2018, to complete the third season in [Burkina Faso, Nigeria and Chad], and carry out an endline molecular markers’ survey in the seven ACCESS-SMC countries, to track trends in parasite resistance to SMC drugs." Malaria Consortium, Summary Update, May 2018, Pg. 1
- 39
- "Malaria Consortium’s three year project, known as ACCESS SMC, will run across seven African countries: Burkina Faso, Chad, Guinea Conakry, Mali, Niger, Nigeria and The Gambia. ACCESS-SMC will be led by Malaria Consortium, with Catholic Relief Services as the primary sub-grantee. It will be supported by London School of Hygiene & Tropical Medicine, Management Sciences for Health, Medicines for Malaria Venture, Speak Up Africa and Centre de Support en Sante International." Malaria Consortium, ACCESS-SMC announcement, May 8, 2014.
- "The ACCESS-SMC partnership:
- Malaria Consortium is leading the ACCESS-SMC project, tracking its impact, managing the procurement of SMC drugs and supporting malaria control programmes to implement SMC in Burkina Faso, Chad and Nigeria.
- Catholic Relief Services is the lead-subrecipient and contributing to tracking the reach and
impact of the project and supporting malaria control programmes to implement SMC in Guinea, Mali, Niger and The Gambia. - London School of Hygiene & Tropical Medicine is generating evidence on drug resistance, strengthening pharmacovigilance and measuring SMC’s public health impact.
- Medicines for Malaria Ventures is supporting manufacturers to develop a child friendly, dispersible formulation and ensuring accurate drug forecasting.
- Management Sciences for Health is measuring the cost of SMC and working with countries to optimize the SMC supply chain.
- Speak Up Africa is creating an integrated health communications and advocacy campaign. The complementary nature of the partnership, which includes non-profit and academic institutions, allows substantial geographic reach and ensures that good evidence will guide future SMC implementation." ACCESS-SMC fact sheet, Pg 2.
- 40
- GiveWell's non-verbatim summary of conversations with Malaria Consortium staff, November 7 and November 9, 2016.
- "The ACCESS-SMC partnership:
- Malaria Consortium is leading the ACCESS-SMC project, tracking its impact, managing the procurement of SMC drugs and supporting malaria control programmes to implement SMC in Burkina Faso, Chad and Nigeria.
- Catholic Relief Services is the lead-subrecipient and contributing to tracking the reach and impact of the project and supporting malaria control programmes to implement SMC in Guinea, Mali, Niger and The Gambia." ACCESS-SMC fact sheet, Pg 2.
- 41
- See Table 7, ACCESS-SMC multi-country cost analysis, January 2017, Pg 19.
- See "Costs" sheet, GiveWell SMC cost per treatment estimate, November 2017.
- Malaria Consortium shared the following source with us to help explain its staff structure: ACCESS-SMC, organizational structure.
- 42
See this spreadsheet for regions supported and children targeted with GiveWell-directed funds from 2017-19.
- 43
- In 2017, GiveWell-directed funds supported coverage surveys and enhanced supervision and monitoring for Malaria Consortium's ACCESS-SMC work:
- "Two dimensions of support were prioritized under the funding framework provided by Good Ventures through GiveWell recommendations on SMC...Monitoring and evaluation support, specifically though the execution of multiple coverage surveys and enhanced in-process monitoring (including in ACCESS-SMC areas)." Summary framework of GiveWell / Good Ventures funding toward Malaria Consortium, Pg. 1
- "[G]aps have been identified in a number of M&E areas, due to a phasing out of support for established activities (such as coverage surveys) and/or because of quality assurance gaps identified during the 2015 and 2016 seasons." Summary framework of GiveWell / Good Ventures funding toward Malaria Consortium, Pg. 3
- "GiveWell / Good Ventures funding supported four coverage surveys in Nigeria and Burkina Faso, and three surveys in Chad (after cycles 1, 3 and at the end of the round). As mentioned above, coverage surveys were not carried [out in] Guinea Bissau." Malaria Consortium, Summary Update, May 2018, Pg. 7.
- "All contracts with independent research institutions were signed in July. To finalize the independent evaluation framework started under ACCESS-SMC, LSHTM was also contracted in the role of independent technical advisory organization, supporting the revision of the evaluation protocols (whose amendments required a revised ethical approval), additional technical supervision of field surveyors, and the analysis and interpretation of results in collaboration with Malaria Consortium technical team." Malaria Consortium, Summary Update, May 2018, Pg. 7.
- "Seven temporary staff were recruited in Chad and, 43 (one per LGA) Nigeria, in order to improve supervision and monitoring and make sure that administrative data received is reflective or the real distribution process in the field. In addition, in Nigeria, independent monitors piloted in-process monitoring during one cycle." Malaria Consortium, Summary Update, May 2018, Pg. 8.
- "[Chad] was support[ed] in terms of extra staff (five field officers for which there was not available budget under ACCESS-SMC) and additional budget for supervision logistics." Malaria Consortium, Summary Update, May 2018, Pg. 6.
- "New SMC child cards (which are normally distributed for multiple years) were printed in 2017, which included a unique identifier of 7 or 8 figures. [...] The cost of reproducing these tools was not fully represented in the UNITAID budget, and not budget expansion was agreed. … [R]esults were mixed, and only in Burkina Faso it appeared that CHWs/volunteers had enough literacy/numeracy to perform the task so that numbers were readable and, mostly, correct. As the difficulties in manual entry (writing) by CHWs became clear in Chad and Nigeria, the exercise was scrapped." Malaria Consortium, Summary Update, May 2018, Pg. 8.
- 2018: “Two key dimensions of support were prioritized in 2018 under the GiveWell-directed funding framework for SMC:...Supervision, monitoring and evaluation support, specifically through enhanced supervision, conducting a range of surveys to determine intervention coverage and the review of the evaluation framework for SMC with a focus on impact assessment.” Malaria Consortium, Summary Narrative Report 2018, Pgs. 1-2.
- 2019: "Malaria Consortium works with governments and implementation partners to deliver the following SMC intervention components:
f) Supervision, monitoring and evaluation
During the SMC campaign, supervision is provided by salaried, facility-based health workers, with support from district, regional and central-level supervisors, as well as Malaria Consortium staff. Administrative monitoring data, including SPAQ doses provided and adverse events, are collected by community distributors on tally sheets, which are compiled by health workers and reported to the district level. In addition to carrying out supervision visits, Malaria Consortium supports supervision and monitoring of the SMC campaign by contributing to the development and printing of tools such as the tally sheets used by community distributors to record doses provided, as well as the interpretation of results.
To draw robust conclusions on program coverage, Malaria Consortium routinely conducts household surveys using lot quality assurance sampling (LQAS) methodology following SMC cycles 1 to 3. The surveys are designed to rapidly assess whether areas have reached a coverage threshold of 80% and determine urgent actions for improvement during subsequent cycles, while also providing an estimate of country- or state-level coverage. To evaluate coverage at the end of the annual campaign, we conduct a representative end-of-round household survey following completion of SMC cycle 4, which encompasses a wider range of variables, including quality of program implementation. All surveys are conducted by independent research firms to reduce bias. Stock consumption and management data collected at health facilities and at district and national warehouses are further sources of monitoring and evaluation (M&E) data." Pg. 6, Malaria Consortium, 2019 SMC narrative report.
- In 2017, GiveWell-directed funds supported coverage surveys and enhanced supervision and monitoring for Malaria Consortium's ACCESS-SMC work:
- 44
- Malaria Consortium, comments on a draft of this page, October 2020.
- See table 9, page 17 of Malaria Consortium, 2019 SMC narrative report.
- 45
- Malaria Consortium emails (unpublished), November 23, 2016.
- Work not discussed above includes some work supported by a "one-off" grant "to implement SMC in six LGAs in the States of Katsina and Jigawa." Summary framework of GiveWell / Good Ventures funding toward Malaria Consortium, Pg. 1
- [Under "Scale up – plans and possibilities":] "In Nigeria in 2014 (9.2 million children) Malaria Consortium will target 500,000 children in Katsina and Jigawa state (CHAI will also implement in Kano state if funding can be obtained)," Slide 22, Malaria Consortium, SMC presentation, May 6, 2014.
- "Malaria Consortium has already started working with the Ministry of Health to roll out SMC in Katsina through its management of SuNMaP (the DFID-funded Support for the National Malaria Programme) and with support from the Bill & Melinda Gates Foundation. SuNMaP will also support the roll out of SMC across Jigawa state and Malaria Consortium has plans to extend the activity to additional states in due course." Malaria Consortium website, "Seasonal Malaria Chemoprevention".
- 46
- See this spreadsheet, Sheet "Past spending by category, Jan-Dec 2019."
- This analysis is focused on the part of Malaria Consortium's SMC work that is funded entirely or in part by philanthropic funding, primarily GiveWell-directed funding. In 2019, Malaria Consortium received the following additional contributions to the SMC programs it supports with GiveWell-directed funding:
- $880,000 from the US President's Malaria Initiative (PMI), as an in-kind contribution of SMC drugs for use in Zamfara State, Nigeria
- $204,133 from the UK government's Department for International Development (DFID) for operational costs of SMC implementation in five LGAs in Jigawa State, Nigeria
- $1,760 from Vitamin Angels, as an in-kind contribution of vitamin A supplements for research in Nigeria on co-implementation of SMC and vitamin A distribution (see this spreadsheet for more details)
- See Malaria Consortium, 2019 SMC narrative report, Table 16, Pg. 32.
- We exclude Malaria Consortium's SMC work that is exclusively funded by institutional funding, specifically the Global Fund to Fight AIDS, Tuberculosis, and Malaria, which funded Malaria Consortium's work in eight LGAs in Nigeria. "Malaria Consortium implemented SMC with funding from the Global Fund in four LGAs in Katsina and four LGAs in Yobe. No philanthropic funding was used in those areas." Pg. 17, Malaria Consortium, 2019 SMC narrative report.
- 47
"Three implementation research studies were implemented across Malaria Consortium’s SMC program in 2019." Malaria Consortium, 2019 SMC narrative report, Pg. 26. See this spreadsheet for more details.
- 48
"With support from Malaria Consortium’s global external relations team, we ran several key communications activities in 2019 to provide the latest information to our partners, donors and the general public. This included five blogs and three news pieces for the Malaria Consortium website, publishing materials on our social media channels, and four email newsletters to an audience of more than 1,500 philanthropic donors. We also improved the SMC-dedicated landing page on our website and hired a photographer to capture images of the SMC campaign in Burkina Faso, which have been used across all of our communication channels.
From an advocacy perspective, key activities focused on strengthening relationships with global SMC stakeholders, most notably WHO." Malaria Consortium, 2019 SMC narrative report, Pgs. 24-25.
- 49
See this spreadsheet, Sheet "Past spending by category, Jan-Dec 2019", Rows 20-22.
- 50
Cost analysis of the seasonal malaria chemoprevention project in Katsina state, Nigeria. For example, see Tables 4 and 5, Pgs 20-21.
- 51
In 2016, the breakdown of direct costs for the ACCESS-SMC program was: 37% to procuring SMC drugs, 37% to delivery of drugs, 16% to staff (including staff at partner organizations), 5% to "direct common costs" (we're not sure what this refers to), and the remaining 5% to a variety of smaller activities. ACCESS-SMC, 2016 direct costs
- 52
- 2017:
- Between May 2017 and February 2018, Malaria Consortium spent about $3.8 million of the about $5 million in GiveWell-directed funds that it had received at that time.
- See Malaria Consortium, Summary Update, May 2018, "Grand total estimate" under column "Budget," Annex I, Pg. 10.
- Of the $3.8 million, Malaria Consortium spent about $2 million (54%) on SMC delivery (excluding drug procurement and delivery), about $0.7 million (19%) on monitoring, about $0.5 million (14%) on drug procurement and delivery, and about $0.5 million (13%) on management, coordination, and overhead costs. See this spreadsheet, Sheet "Past spending by category, May 2017 - Feb 2018."
- We note that these funds complemented funding from the ACCESS-SMC grant and are not representative of the full cost breakdown of Malaria Consortium's SMC work in 2017. "The original ACCESS-SMC grant was expected to end on August 31st [2017], but Malaria Consortium secured a cost extension up to February 28, 2018, to complete the third season in [Burkina Faso, Nigeria and Chad], and carry out an endline molecular markers’ survey in the seven ACCESS-SMC countries, to track trends in parasite resistance to SMC drugs." Malaria Consortium, Summary Update, May 2018, Pg. 1.
- Between May 2017 and February 2018, Malaria Consortium spent about $3.8 million of the about $5 million in GiveWell-directed funds that it had received at that time.
- 2018:
- Between January and December 2018, Malaria Consortium spent about $15.1 million in GiveWell-directed funds.
- Of the $15.1 million, Malaria Consortium spent about $4.8 million (32%) on SMC drugs and other commodities, about $4 million (26%) on SMC implementation, about $2 million (13%) on monitoring, evaluation, and advocacy, about $1.5 million (10%) on staff costs, about $1 million (7%) on planning and drug procurement, and about $1.8 million (12%) on in-country and headquarters offices and other indirect costs. See this spreadsheet, Sheet "Past spending by category, Jan-Dec 2018."
- The ACCESS-SMC project ended in early 2018, so our understanding is that this spending includes the majority of costs to operate Malaria Consortium's SMC programs in its targeted areas for the remainder of 2018. "The original ACCESS-SMC grant was expected to end on August 31st [of 2017], but Malaria Consortium secured a cost extension up to February 28, 2018, to complete the third season in [Burkina Faso, Nigeria and Chad], and carry out an endline molecular markers’ survey in the seven ACCESS-SMC countries, to track trends in parasite resistance to SMC drugs." Malaria Consortium, Summary Update, May 2018, Pg. 1.
- Between January and December 2018, Malaria Consortium spent about $15.1 million in GiveWell-directed funds.
- 2017:
- 53
- Diego Moroso, Project Director for ACCESS-SMC at Malaria Consortium, email to GiveWell, August 14, 2017.
- Malaria Consortium, comments on a draft of this page, October 2019.
- 54
- Some additional details on this activity: "All drugs procurement is centralized at our Kampala office for all countries. We require countries to carry out a revised quantification every year based on consumptions and stocks from previous years, consolidate orders, recruit procurement agent, negotiate pricing points with Guilin (and in the future, more pharma companies we hope), we monitor production and shipment schedules and make sure country teams are aware of when drugs come / delays, etc. Countries are responsible for providing us with all the relevant import requirements to make sure that all documentation is ready when drugs arrive. They then work out with local clearing agents the custom procedures, tax waivers, etc, and proceed with whatever logistics options / approaches are relevant in their countries (from direct delivery to peripheral health units as in Nigeria, to mixed approaches involving MoH storage and MC / CRS transport, to full contracting with MoH-mandated central pharmacy – depends on context)." Diego Moroso, Project Director for ACCESS-SMC at Malaria Consortium, email to GiveWell, August 14, 2017.
- Malaria Consortium's head office is now located in the UK. Malaria Consortium, comments on a draft of this page, October 2019.
- 55
This may include "training materials, monitoring & evaluation forms, bags, t-shirts etc. used by CDs, water and cups for SMC administration." Malaria Consortium, comments on a draft of this page, October 2019.
- 56
- Some additional details on this activity: "Malaria Consortium developed all original training material for ACCESS-SMC [...] We could say that now the materials are pretty settled, with only minor changes / improvement happening yearly or less, based on experience from the field. We co-organize central trainings with [National Malaria Control Programs], and we then support and oversee cascade trainings (always in joint MC/NMCP teams – CRS teams in former CRS countries under ACCESS-SMC)." Diego Moroso, Regional Project Director for ACCESS-SMC at Malaria Consortium, email to GiveWell, August 14, 2017.
- "We do continue to revise tools – mainly with a view to simplifying them, adapting to changing guidelines and harmonizing with other implementers." Malaria Consortium, comments on a draft of this page, October 2019.
- 57
Malaria Consortium, comments on a draft of this page, October 2020.
- 58
See the most recent version of the model here.
- 59
Malaria Consortium, comments on a draft of this page, October 2017
- 60
For example, see:
- "Malaria is still a serious public health concern in Nigeria, with fevers presumed to be malaria accounting for 60 percent of outpatient visits to health facilities, 30 percent of childhood deaths, 25 percent of deaths in children under one year and 11 percent of maternal deaths. In northern Nigeria, where malaria transmission is highly seasonal, malaria prevalence is also comparatively higher than other areas during the rainy seasons. The implementation of SMC in Katsina is specifically intended to reduce mortality in children under five living in areas with seasonal malaria, and strengthen health systems at the state and national levels." Malaria Consortium, Project Brief: Seasonal malaria chemoprevention, Katsina, Pg 1.
- Under "Why Katsina State" in Malaria Consortium, SMC presentation, May 6, 2014:
- "Katsina State is within the Sahel Region; rainy season and peak malaria incidence from July to October
- 2012 estimated population of 6,916,641
- 1,383,328 under-5 years
- 600,281 cases of malaria (2008)
- 4,103 malaria related deaths
- Katsina overall under-5 mortality rate 180 per 1,000 live births
- Also, Malaria Consortium told us that “eligible districts are established by the National Malaria Programmes in the countries applying the WHO rainy season seasonality rules.” Malaria Consortium emails (unpublished), November 23, 2016.
- 61
See the most recent version of the model here.
- 62
In 2017, Malaria Consortium supported one region in Guinea Bissau with SMC but did not conduct a coverage survey. "Due to the size of the country, and the expected transient nature of Malaria Consortium support there, it was decided not to include any coverage surveys in Guinea Bissau." Pg. 6, Malaria Consortium, Summary Update, May 2018.
We do not view this as a major limitation, as a relatively small portion of Malaria Consortium's spending in 2017 went to this program. See this spreadsheet, Sheet "Past spending by category, May 2017-Feb 2018," row "Guinea Bissau 'GiveWell Districts'."
- 63
See the most recent version of the model here, "Malaria Consortium" sheet, line "Misappropriation without monitoring results."
- 64
Malaria Consortium started conducting post-cycle surveys in 2017 in order to determine whether surveys conducted closer to the time of SMC delivery would yield different coverage rates than surveys conducted at the end of the round (four or more months after the first SMC cycle of the season) and to identify and respond to program delivery issues during SMC campaigns.
- "In 2017, due to concern about methodology and especially linked to potential recall bias, we reorganized the framework as a series of 4 surveys post each cycle." Diego Moroso, Regional Project Director for ACCESS-SMC at Malaria Consortium, email to GiveWell, May 8, 2018.
- "In previous years, only one coverage survey was conducted at the end of the annual SMC round to measure coverage achieved by Malaria Consortium’s SMC program. This meant that administrative data had to be relied on to gauge coverage while the campaign was ongoing. As gathering and compiling administrative data is a time-consuming process, this limited the program’s capacity to take evidence-based corrective action and make adaptations to the intervention during the campaign. For this reason, Malaria Consortium decided to implement coverage surveys following each of the four SMC cycles in each of the three implementation countries." Malaria Consortium, 2018 SMC Coverage Report, Pg. 12.
- "End-of-cycle (EoC) surveys employing the lot quality assurance sampling methodology following cycles 1 to 3 to enable implementing teams to identify areas of low coverage and rapidly take corrective actions to improve SMC delivery in subsequent cycles." Malaria Consortium, 2019 coverage report, Pg. 4.
Malaria Consortium reports that post-cycle surveys conducted in 2019 enabled it to identify and respond to program delivery issues in one health district in Chad and several LGAs in Nigeria; we do not know the details of these cases. "Were there any instances in 2019 in which results from post-cycle coverage surveys identified issues that were addressed in later cycles? Strengthening decision making based on end-of-cycle survey results has been a key project our M&E team has been working on over the last few months. So far, this has been handled less systematically, with country teams taking action at their discretion. In Chad, a capacity building session was organised with district health authority staff in [a] poorly-performing health district to emphasise the SMC administration protocol. Similar exercises were conducted in various LGAs in Nigeria. In 2020, we are setting up a system to assess 15 indicators using LQAS with specific decision criteria for each, to identify specific issues in quality of SMC delivery by supervision are[a], with a requirement (COVID-19 permitting) to follow up locally about the specific issues." Malaria Consortium, Answers to GiveWell's questions, July 2020 (unpublished).
- 65
"Comprehensive end-of-round (EoR) surveys following the completion of the SMC round (that is, after cycle 4) to assess SMC performance across all four monthly cycles." Malaria Consortium, 2019 coverage report, Pg. 4.
- 66
See the most recent version of the model here, "Malaria Consortium" sheet, line "False monitoring results."
- 67
"Post-cycle surveys using lot quality assurance sampling (LQAS) methods were conducted following Cycles 1, 2 and 3...LQAS surveys are a comparatively simple and operationally feasible way of collecting data from randomly selected supervision areas and are designed to assess whether or not the minimum expected coverage of 80% was achieved. Aggregating data at the national or state level results in a reasonably precise measure of coverage." Malaria Consortium, Summary Narrative Report 2018, Pg. 8.
This approach is intended to be quicker and cheaper than typical coverage surveys, as data collectors receive less training and ask fewer questions at each household. Malaria Consortium told us that it expected to use the post-cycle results to identify and respond to implementation issues. "An LQAS framework can be done quickly by personnel with basic training, using low inputs. Results from such enquiries can be generated in real time through less complex analysis. In other words, LQAS is a valid sampling approach for rapid surveys, which offers managers action oriented information on the spot...The proposed monitoring framework combines the advantages of both approaches. The plan would be to carry out a LQAS survey at the end of Cycles 1, 2 and 3...and then to deploy a full coverage survey at the end of Cycle 4. This methodology offers the advantage of 1) quick classification of defined supervision areas in real time, to address implementation issues as they arise and improve performance, and 2) the opportunity for independent surveyors to assess coverage with an acceptable margin of error." Diego Moroso, Regional Project Director for ACCESS-SMC at Malaria Consortium, email to GiveWell, May 8, 2018.
- 68
“In the context of public health programs, LQAS subdivides program implementation areas into smaller functional areas (e.g. wards or health facility catchment areas), which are referred to as “Supervision Areas” (SAs), with the aim of classifying whether SAs have or have not reached a predetermined coverage standard. The LQAS method results in the need for a relatively small sample per SA as it does not attempt to construct a precise estimate of population parameters (e.g. standard errors). Although this limits interpretation of findings at the SA level, the smaller sample size allows for surveys to be rapidly completed to inform actions for program improvements (for example, between monthly cycles in the case of SMC).” Pg. 15, Malaria Consortium, 2019 coverage report.
- 69
See this spreadsheet, "Methods" sheets, rows 4.
- 70
See this spreadsheet, "Methods" sheets, rows 4.
- 71
"The post-round survey report from Nigeria describes a procedure in which data collectors spin a bottle to randomly select households to be interviewed. Was this randomization procedure used to select households for all surveys? How did data collectors select a child in each household?
The spin-the-bottle method was used in all three countries to select households. The protocol for selecting children in each household only specified that a child should be selected randomly, but left the selection method up to the data collector. In 2020, we are using a different data collection platform, which has an in-built randomisation feature for selecting children." Malaria Consortium, Answers to GiveWell's questions, July 2020 (unpublished).Description of "spin-the-bottle" method in Nigeria: "For compound selection at the community level, a central mosque was located at which a bottle was spin to select the part in the community for the selection of the first compound. Two electronic data collection applications were used for data collection - SurveyCTO for compound and child selection, and Magpi for the administration of the survey questionnaire. The GPS of the starting point was recorded. All the compounds along the path of the bottle direction were listed. The first compound was then randomly selected. Using systematic methods sampling, seven (7) compounds were selected within the community/settlement using skipping pattern at interval of two compounds." Pg. 14, Malaria Consortium, Nigeria end-of-round coverage survey report, 2019 (unpublished).
- 72
"The steps followed for selecting respondents and children in 2019 was as follows (using Nigeria as an example):
a) On reaching compounds using the randomisation procedures described in the coverage report, data collectors opened a survey software called SurveyCTO on their devices.
b) Data collectors made a list of all children aged under 120 months of age in the compound and determined whether each child received SP and the first dose of AQ from community distributors. In the Nigeria End-of-Round Report (Appendix 6 of the Coverage Report), estimates of SMC coverage use all children aged 3 to 59 months listed through this process as the denominator.
c) SurveyCTO was then used to randomly select two children from among the listed children aged 3 to 59 months. The names of the two children were noted on a piece of paper. Random selection did not consider the treatment status (whether they had received SP and the first dose of AQ from community distributors on Day 1), i.e. probability of selection was independent of treatment status.
d) The data collector then switched to another survey software called Magpi. Questions were asked about the first selected child, based on the treatment status determined and recorded in SurveyCTO. If the child was not treated, data collectors asked questions 10 to 18 (as per Appendix 3 – End-of-Round questionnaire). If the child was treated, data collectors skipped to question 19. This group of children was designated as the ‘non-treatment group’, as only respondents in this group were asked questions regarding reasons for non-treatment, if applicable.
e) For the second child aged 3 to 59 months selected randomly by SurveyCTO, data collectors started with question 19. If the child was not treated, no further questions were asked and data collectors closed the interview. If the child was treated, data collectors proceeded to ask the remaining questions on the questionnaire. This group of children is designated as the ‘treatment group’.
Children selected and referred to in Questions 10 and 19 are effectively independent random samples, independent of treatment status. The proportions of children found to be treated in the ‘treatment’ and ‘non-treatment’ groups were very similar (around 85%). In our 2019 Coverage Report, estimates of SMC coverage are based on the ‘treatment group’ only. This was considered most appropriate as subsequent estimates of proportion of children who received the remaining two doses of AQ on days 2 and 3 are calculated using the same analytic sample.
The coverage estimates in the 2019 Coverage Report are effectively based on a sample in which data were obtained for only one child per compound. Although likely to be negligible, there may be the possibility that the coverage estimates obtained are biased as they over-represent eligible children living in compounds with fewer children. It was not possible to determine the number of children per compound before it was visited. The coverage estimates are based on the assumption that compounds have similar number of eligible children living in them." Malaria Consortium, Answers to GiveWell's questions, September 2020 (unpublished). - 73
See this spreadsheet, "Methods" sheets, rows 6.
- 74
Examples:
- 75
"Generic questionnaires for both types of survey were initially developed in English and translated into French for use in Burkina Faso and Chad. Questionnaires were further adapted by Malaria Consortium staff in each country according to the specific context, for example by changing terminology used to reflect differences in local administrative units or program terminology." Pg. 14, Malaria Consortium, 2019 coverage report.
- 76
"Interviews were conducted in local languages using questionnaires provided by Malaria Consortium, with data collectors translating from the French or English questionnaire on the spot and assigning responses to pre-defined answer categories in Magpi." Pg. 15, Malaria Consortium, 2019 coverage report.
- 77
Caregivers report on four cycles of SMC, with three days of administration in each cycle.
- 78
In some cases, post-round surveys have been delayed, lengthening the recall period for responses. This was the case for both the 2018 and 2019 post-round surveys in Chad, which were conducted three months after the end of the relevant SMC rounds.
- "For example, in Chad...finding an external firm to coordinate the EoR survey took much longer than expected, resulting in the survey only being conducted in January 2019, several months after the end of the 2018 SMC round. This is much later than recommended for coverage surveys and increases the risk of recall bias." Malaria Consortium, 2018 SMC Coverage Report, Pg. 20.
- "It was also problematic in Chad, where the EoR survey was only conducted in January due to challenges in engaging a suitable supplier." Pg. 41, Malaria Consortium, 2019 coverage report.
- 79
See this spreadsheet, "Results" sheets.
- 80
- Our understanding is that CDs are expected to record the first dose of each cycle and the date they gave the dose on a family's record card and that the family is expected to record all other doses. We have seen a few versions of templates for SMC record cards. The latest version that we have seen (from 2016) is here: SMC Record Card Template 2016.
- "Does the child have an SMC card? (Yes or No)...Ask to see blister: present? (Y or N)...Are there tablets remaining in the blister?" Malaria Consortium, 2018 end-of-round survey report, Nigeria, Pgs. 54-57.
- 81
Card retention in Burkina Faso was 88% in 2017, 81% in 2018, and 68% in 2019; in Chad, it was 44% in 2017, 70% in 2018, and 62% in 2019; and in Nigeria, it was 30% in 2017, 37% in 2018, and 68-90% in 2019.
- See this spreadsheet, sheet "Results: Analysis (2015-2019)," rows 73 and 127.
- "According to data obtained by EoR data collectors from SMC Child Record Cards, in Burkina Faso 3,251 of 4,896 children sampled, or 67.9% (95% CI: 61.2–74.0; weighted data from 23 health districts) had record cards…In Chad, SMC Child Record Cards were found for 1,634 of 2,620 children, or 62.4% (95% CI: 60.5–64.2) of the sample with available data…In Nigeria, the weighted proportions of children with SMC Child Record Cards ranged from 67.8% (95% CI: 64.7–70.8) in Sokoto to 90.0% (95% CI: 82.4–96.7) in Yobe." Pg. 30, Malaria Consortium, 2019 coverage report.
With no SMC card present, it is more difficult to check caregivers' responses. We note, however, that in the 2017 coverage surveys, which reported on the percentage of children for whom caregiver responses agreed with SMC cards, coverage rates measured from these sources were generally similar, with agreement ranging from 86% to 99%. See this spreadsheet, sheet "Methods (2015-2018)," column "Percentage of children for whom caregiver report agrees with SMC card."
In past surveys, the greater of the number of doses noted on the SMC card or reported by the caregiver was recorded as the number of doses the child received; since 2018, Malaria Consortium has primarily used caregiver responses to measure coverage, due to low retention of SMC cards.
- "SMC record cards greatly improve accuracy. However, caregivers do not always retain the cards; in these cases caregiver recall is the only source of information. In addition, SMC record cards are not always fully completed by health workers, meaning that the cards are not necessarily reliable. To mitigate the problem of unreliability, ACCESS-SMC researchers ask the caregiver to remember the number of treatments their child had without looking at the record card, then check the caregiver's answers against the card. Agreement has been generally good but where there is a discrepancy the interviewer tries to resolve this; if this cannot be done, the number of treatments indicated on the card is used unless the caregiver states a larger number, in which case the caregiver’s report is used, as it is known that cards can be under-completed." GiveWell's non-verbatim summary of a conversation with Paul Milligan and Diego Moroso, August 2, 2017, Pg. 5
- "In 2018, we only reported self-reported coverage. No adjustments/reconciliations were made. Data on SMC card retention and coverage according to SMC cards is available...but we believe that due to low retention rates, self-reported coverage is the most meaningful indicator." Malaria Consortium, comments on a draft of this page, October 2019
- "Coverage can also be calculated on the basis of SMC Child Record Cards, which are given to caregivers by community distributors the first time they administer SPAQ to a child each season. Caregivers are then asked to record administration of AQ doses 2 and 3 and to retain the card. Caregivers should produce the card during subsequent cycle visits and information on the card is updated. In previous years, it has been observed that SMC Child Record Cards have represented an unreliable data source, as their retention by caregivers can be low in some areas, and information recorded by caregivers on doses administered to children at home after distributor visits may be inconsistent…While this report gives consideration to data from SMC Child Record Cards as a method for estimating coverage, this will be presented as a secondary coverage indicator." Malaria Consortium, 2019 coverage report, Pg. 13.
In 2017, some coverage surveys also gathered data on the proportion of caregivers who had retained the blister pack that contained the SMC medicines, another possible check on caregivers' responses. However, in most of the examples we've seen, a substantial number of caregivers did not retain the blister packs:
- In Burkina Faso in 2017, blister packs were retained in 24%, 56%, and 59% of cases in cycles 1, 2, and 3 respectively. Malaria Consortium, Summary of Results for Cycle 1, Burkina Faso 2017 Pg. 2; Malaria Consortium, Summary of Results for Cycle 2, Burkina Faso 2017 Pg. 3; Malaria Consortium, Summary of Results for Cycle 3, Burkina Faso 2017 Pg. 3.
- In Chad in 2017, blister packs were retained in 14% and 32% of cases in cycles 1 and 3 respectively. Malaria Consortium, Summary of Results for Cycle 1, Chad 2017 Pg. 3; Malaria Consortium, Summary of Results for Cycle 3, Chad 2017, Pg. 3.
- In Nigeria in 2017, blister packs were retained in 100% of cases in cycles 2 and 3. We think the retention results in Nigeria seem implausibly high, but we have not vetted these figures. Malaria Consortium, Summary of Results for Cycle 2, Nigeria 2017 Pg. 3; Malaria Consortium, Summary of Results for Cycle 3, Nigeria 2017, Pg. 3.
The 2018 and 2019 coverage surveys measured but did not report on the proportion of caregivers who had retained blister packs; we have not requested this information.
- 82
See this spreadsheet, "Methods" sheets, rows 10.
- 83
In Nigeria in 2018, post-cycle surveys were conducted by data collectors recruited by state-level health authorities, who also implemented SMC, and these data collectors may have non-randomly selected villages to survey. This may have biased data collection in two ways: 1) data collectors may have been influenced to report more positive results, and 2) because data collectors were knowledgeable about how SMC was delivered, they may have chosen to survey villages that were more likely to have received SMC. This may have biased the coverage estimates produced by these post-cycle surveys upward.
- "In 2018, post-cycle coverage was found to be consistently above 90%, often hovering very close to 100% in all states. However, post-cycle survey results should be interpreted with caution, as several methodological issues were documented and there were concerns over researchers being recruited from among state-level health authority staff. The post-round coverage survey found generally lower coverage figures, ranging from 92% in Katsina to 95% in Jigawa, which is likely a better reflection of actual coverage." Malaria Consortium, Summary Narrative Report 2018, Pgs. 7-8.
- "The main challenge related to researchers being selected by political LGA authorities. This increases safeguarding risk and may introduce bias because state authorities have a vested interest in reporting positive implementation results. Another limitation arises from researchers’ familiarity with implementation plans. If frontline implementers focus on “easy-to-reach” areas within a supervision area (the unit of randomization) and researchers, aware of implementers’ activities, simply follow in their footsteps, this may lead to an overestimation of coverage." Malaria Consortium, Summary Narrative Report 2018, Pg. 9.
- "In Nigeria, EoC surveys were carried out by data collectors recruited through state- or LGA-level health authorities, which raises concerns about the impartiality of data collectors, as their employers would have a stake in implementing a successful SMC campaign. There were also concerns that the data collectors, being familiar with the SMC campaign, may have selected villages they knew had been covered by drug distributors." Malaria Consortium, 2018 SMC Coverage Report, Pg. 20.
- "To note: there is no evidence that this actually happened, but we discussed this as a possible limitation in the report." Malaria Consortium, comments on a draft of this page, October 2019.
- 84
- "Chad: The quality of outputs from some of the selected research firms was unsatisfactory and required Malaria Consortium to carry out additional, time-consuming analyses. Despite outsourcing post-cycle surveys to different firms each cycle, deliverables did not meet our expectations, leading us to conclude that local research firms do not have the required capacity and expertise to conduct complex evaluations of coverage and quality." Malaria Consortium, Summary Narrative Report 2018, Pg. 9.
- "We therefore decided to rely on independent surveys conducted by third parties. However, it should be noted that this may pose different challenges. This was particularly the case in Chad, where capacity of local research firms to execute large-scale surveys is relatively low. Due to inability to engage a suitable contractor no cycle 2 EoC survey was carried out. The EoR survey was also delayed as it was necessary to contract the same research firm from Burkina Faso which also conducted that country’s EoR survey." Pg. 41, Malaria Consortium, 2019 coverage report.
- "What caused you to determine that the quality of coverage data from the first post-cycle survey in Chad was poor?...Data collection for the first end-of-cycle survey was slow and a succession of draft reports were incomplete with essential information missing. When reviewing the raw dataset, it was noted that it did not correspond exactly to the survey codebook, which may have been a result of mislabelling of variables. In addition, some variables were missing altogether. The local company that conducted the survey following cycle 3 provided better quality work and the regional firm that was commissioned for the end-of-round survey was very good." Malaria Consortium, Answers to GiveWell's questions, July 2020 (unpublished).
- 85
See this spreadsheet, "Methods" sheets, rows 9.
In 2017, survey team supervisors were instructed to repeat some interviews to ensure that interviewers administered the survey questionnaires properly. "LSHTM sends monitors into the field during surveys to provide technical assistance to research teams. In addition, survey team supervisors are instructed to repeat some interviews to ensure that interviewers are administering the survey questionnaires properly." GiveWell's non-verbatim summary of a conversation with Paul Milligan and Diego Moroso, August 2, 2017, Pg. 4
We have seen data from these repeat interviews in Nigeria in 2017. Approximately 90% of repeat interviews produced the same answers for each of “number of eligible children” and "number of children treated" per household.
- Number of eligible children: (68+31+5)/(68+1+6+31+1+1+5+2) = 90.4%, SMC in Nigeria: Coverage surveys 2017, Pg. 41
- Number of children treated: (40+43+16+4)/(40+3+3+43+1+2+16+1+1+1+4) = 89.6%, SMC in Nigeria: Coverage surveys 2017, Pg. 41
We have not seen data on these audits from other coverage surveys.
- 86
See this spreadsheet, "Methods" sheets, rows 13.
- 87
See this spreadsheet, sheet "Results: Analysis (2015-2019)," cells B211-214.
- 88
See this spreadsheet, sheet "Results: Analysis (2015-2019)," cells B172-D172.
- 89
See this spreadsheet, sheet "Results: Analysis (2015-2019)," cells B99, B147, and B201.
- 90
See this spreadsheet, sheet "Results: Analysis (2015-2019)," rows 141 and 195.
- 91
- For Nigeria in 2018, Malaria Consortium proposes three possible explanations for the inconsistent results: "Again, the EoR survey found slightly lower coverage in Nigeria...This difference could be a result of recall bias, data collectors’ bias or it could be due to questionnaire design." Malaria Consortium, 2018 SMC Coverage Report, Pgs. 22-23. Of these, it appears that bias during data collection may have contributed most to skewing post-cycle results upward:
- "In 2018, post-cycle coverage was found to be consistently above 90%, often hovering very close to 100% in all states. However, post-cycle survey results should be interpreted with caution, as several methodological issues were documented and there were concerns over researchers being recruited from among state-level health authority staff. The post-round coverage survey found generally lower coverage figures, ranging from 92% in Katsina to 95% in Jigawa, which is likely a better reflection of actual coverage." Malaria Consortium, Summary Narrative Report 2018, Pgs. 7-8.
- "The main challenge related to researchers being selected by political LGA authorities. This increases safeguarding risk and may introduce bias because state authorities have a vested interest in reporting positive implementation results. Another limitation arises from researchers’ familiarity with implementation plans. If frontline implementers focus on “easy-to-reach” areas within a supervision area (the unit of randomization) and researchers, aware of implementers’ activities, simply follow in their footsteps, this may lead to an overestimation of coverage." Malaria Consortium, Summary Narrative Report 2018, Pg. 9.
- For Nigeria in 2019, our best guess is that post-round results are again a better indication of actual coverage. The fact that in two consecutive years, post-cycle results have differed substantially from post-round results and have been surprisingly high (over 95% coverage in all cases) makes us concerned that there may have been issues in post-cycle survey implementation in Nigeria that Malaria Consortium has not identified and that biased results upward.
- For Chad in 2019, our best guess is that post-round results are a better indication of actual coverage. Post-cycle surveys were conducted after the first and third SMC cycles in Chad in 2019. Malaria Consortium reports that the first post-cycle survey in Chad was of poor quality, so we have limited confidence in the reliability of those results. The third post-cycle survey found coverage of around 98%, which we find implausibly high.
An additional consideration is that Malaria Consortium told us that it places more weight on post-round results, as post-round surveys are explicitly designed to achieve representative samples, whereas post-cycle surveys are designed to assess whether each program area unit met a target coverage level (see discussion above). This understanding is from Malaria Consortium, conversation with GiveWell, August 20, 2020 (unpublished).
- For Nigeria in 2018, Malaria Consortium proposes three possible explanations for the inconsistent results: "Again, the EoR survey found slightly lower coverage in Nigeria...This difference could be a result of recall bias, data collectors’ bias or it could be due to questionnaire design." Malaria Consortium, 2018 SMC Coverage Report, Pgs. 22-23. Of these, it appears that bias during data collection may have contributed most to skewing post-cycle results upward:
- 92
More detail on the methods is given as follows: "The relative reduction in the number of malaria cases and malaria deaths under five years of age, compared to older age groups, observed when SMC was introduced, was estimated from the number of confirmed cases of deaths in-hospital that were reported in the national Health Management Information Systems, supplemented by data on malaria cases collected from clinic registers in selected health facilities in each country." Malaria Consortium, Annex III: ACCESS-SMC Evaluation, Pg. 3.
- 93
- The data we have seen for Mali, Chad, and Niger is presented on Pg. 91-102 of Malaria Consortium, Annex III: ACCESS-SMC Evaluation.
- "As part of the Unitaid funded 2015-17 Achieving Catalytic Expansion of Seasonal Malaria Chemoprevention in the Sahel (ACCESS-SMC) program, which was led by Malaria Consortium, Milligan et altera from the London School of Hygiene and Tropical Medicine conducted an impact assessment, which explored malaria cases and deaths using reported data from national Health Management Information Systems (HMIS) and sentinel sites (Milligan, 2018). Data abstracted from sentinel sites showed that the estimated average reduction of malaria cases in 2015 and 2016 were 41% and 49% in Burkina Faso; 37% and 25% in Chad; 25% and 25% in Nigeria, respectively." Malaria Consortium, 2019 impact report, Pg. 3.
- 94
"In countries with consistent reporting, introduction of SMC was associated with a reduction of about 50% in the number of malaria deaths in implementation areas." Malaria Consortium, Annex III: ACCESS-SMC Evaluation, Pg. 3.
- 95
- "Data for impact indicators outlined in Table 1 were abstracted from the national HMIS over the years of 2013 to 2018 for Burkina Faso and Chad, and 2017 to 2018 for Nigeria and were retrospectively analysed." Malaria Consortium, 2019 impact report, Pg. 4.
- "To generate estimates of the level of impact of SMC, a Poisson regression model was fitted to the number of cases confirmed by RDT or microscopy reported to health facilities during the SMC distribution months (August, September, October, November) between 2013 and 2018 (or 2017-2018 for Nigeria)." Malaria Consortium, 2019 impact report, Pg. 6.
- 96
"Analysis of HMIS data at the national level for each country overall showed no evidence of impact." Malaria Consortium, 2019 impact report, Pg. 9.
- 97
- "A review of the quality of HMIS data for each country was conducted, which showed varied results between and within countries...Checks for data completeness were conducted for each country, as defined by the proportion of all monthly reports for each indicator available. Briefly, Burkina Faso showed high completeness of data with less than 1% of missing data points for the relevant indicators. Data coming from Chad and Nigeria showed low completeness ranging from 36- 42%. To mitigate potential bias from low completeness, any health facility or district with more than 1 month missing data during the SMC distribution period was excluded...Further checks for data accuracy revealed data entry errors as well as major health data reporting issues. First, common in all countries were obvious data entry errors most likely due to typing errors, entering data into the incorrect field, recording data in the wrong month, etc. The level of data accuracy varied across and within countries. Additionally, the analysis also revealed major data reporting issues that bring into question the overall accuracy of data that is captured in the national HMIS. For example, in Burkina Faso, approximately 30% of the data points reported more malaria cases in children under 5 than there were children seen that month." Malaria Consortium, 2019 impact report, Pg. 7.
- "However, as previously discussed, the quality of data, the limitations of HMIS data reporting, and the inability to adjust for major factors affecting malaria transmission contribute to noise in the analysis and result in inaccurate estimates of effect...With regards to quality of impact data, in this analysis, we sought to analyse impact indicators available through the national HMIS from Burkina Faso, Chad, and Nigeria. Overall, the analysis indicated that accurate health data reporting is still a point of improvement for health systems and limitations to the data must be considered when interpreting results from national HMIS. Inaccurate data with no means of verification contribute to noise in the analysis and result in either dampening or amplification of estimated effects." Malaria Consortium, 2019 impact report, Pg. 9.
- 98
- "While we are aware of the limitations of HMIS data and we have started using other sources of data to determine impact, we will continue to analyse HMIS data for 2 reasons: 1) It is commonly used by governments and bilateral/multilateral organisations to track progress/impact of health programmes; 2) in the long-term, it is the most sustainable/affordable source of impact data and we aim to contribute to strengthening HMIS in the long run." Malaria Consortium, comments on a draft of this page, October 2019.
- "It’s really about sustainability. We aim to avoid creating new or parallel systems wherever possible. In the longer-term, HMIS should serve as the main source for impact data for health programmes. Surveys, cohort studies etc. are expensive and not sustainable as a routine source of impact data. We see it as part of our remit to contribute to the strengthening of existing health system structures and we will continue to use HMIS data for our analyses." Malaria Consortium, comments on a draft of this page, October 2020.
- 99
- "Efficacy of SMC treatments will be measured using the case control approach. Malaria cases, and controls who do not have malaria, will be recruited concurrently, and the dates of the doses of SMC they received noted. The efficacy of SMC can then be calculated as a function of the time since treatment using case-control analysis. It is essential that dates of SMC doses are accurately documented, and that malaria cases are parasitologically confirmed. Controls will be selected from the community, in the neighbourhood where the case lived at the time they had malaria. Trained fieldworkers will collect information about cases and controls, and make home visits to record bednet use and other household factors that may act as confounders. Microscopy will be used to confirm cases and to measure parasite density. Controls will be confirmed to be negative for P.falciparum, by RDT." Malaria Consortium, Annex III: evaluation of seasonal malaria chemoprevention, Pg 10.
- "The dataset from Nigeria did not pass quality control when compared against scanned forms, and are being independently re-entered", Malaria Consortium, Annex III: ACCESS-SMC Evaluation, Pg. 75.
- 100
Malaria Consortium, Annex III: ACCESS-SMC Evaluation, Pg. 76.
- 101
"820 cases and 1,637 controls were recruited in 2015, and 1,433 cases and 2,867 controls in 2016. SMC was associated with an 89% reduction in malaria incidence for 4 weeks after treatment, and 62% from 5 to 6 weeks after treatment, compared with children who had not received SMC or whose last dose was more than 6 weeks before", Malaria Consortium, Annex III: ACCESS-SMC Evaluation, Pg. 2.
- 102
"SMC at scale will exert a selection pressure for drug resistance, which is expected to lead to progressive loss of efficacy, but it is not known how quickly this will become a problem." Malaria Consortium, 2018 drug resistance surveys preliminary report, Pg. 5.
- 103
- "Resistance to sulfadoxine-pyrimethamine (SP) and amodiaquine (AQ) is associated with specific gene mutations in the malaria parasite. Monitoring the prevalence of these markers, in malaria cases health facilities and in P. falciparum carriers in the general population, permits early warning of emerging problems with drug resistance. This progress update is on monitoring P. falciparum resistance markers. The objective of the molecular monitoring through the ACCESS-SMC project was to establish a baseline for monitoring the prevalence of the markers across the subregion using standardised methods, and to determine if there have been any important changes in the prevalence of these markers after two years of SMC at scale. [...] The baseline surveys were done at the end of the 2015 transmission season in all seven countries. (In 6 countries, sampling was done in areas which had not started SMC but would implement the following year. In The Gambia, sampling was in an area where SMC had been implemented for two years). In each country, one locality was chosen, blood samples were collected from approximately 2000 children under 5 years of age and 2000 individuals 10-30 years of age per country, taken onto filter paper and shipped to London to the LSHTM laboratories for analysis. The older age group was included because they would not be treated with SMC drugs, assessment of trends in the prevalence of markers of resistance in this group therefore allows us to determine whether SMC is leading to changes in the circulating parasite population." ACCESS-SMC, Progress update - Resistance monitoring, Pg. 3
- "The baseline surveys, before scale-up of SMC, showed very low frequencies of mutations associated with SP and AQ resistant genotypes." ACCESS-SMC, Progress update - Resistance monitoring, Pg. 3
- 104
"To determine whether there have been any changes in the frequency of molecular markers of resistance to SMC drugs after two years of SMC at scale, surveys were conducted in each of the 7 ACCESS-SMC countries after the 2017 transmission season, in the same locations and using the same sample size and sampling approach as the baseline survey." Malaria Consortium, 2018 drug resistance surveys preliminary report, Pg. 5.
- 105
- "Thus overall, mutations associated with amodiaquine resistance have not increased in prevalence and the prevalence of the combination pfmdr1-86Y with pfmdr1-184Y occurred with a prevalence of 1.6% and the combination of pfmdr1-86Y and pfmdr1-184Y with pfcrt-CVIET occurred with a prevalence of 0.89% in the 10-30 year age group. The mutations associated with resistance to SP, increased in prevalence between 2016 and 2018. In the 10-30 year age group the prevalence of the dhfr triple mutation increased from 81% to 90%, and the overall prevalence of the dhps 540E mutation increased almost 5-fold." Malaria Consortium, 2018 drug resistance surveys preliminary report, Pg. 9.
- "This is not surprising considering the time that SP has been available and used as an antimalarial in Africa." Malaria Consortium, comments on a draft of this page, October 2019.
- 106
"No samples carried genotypes associated with resistance to both SP and amodiaquine." Malaria Consortium, 2018 drug resistance surveys preliminary report, Pg. 4.
- 107
See the most recent version of the model here, "Malaria Consortium" sheet, line "Drug resistance."
- 108
See the most recent version of the model here, "Malaria Consortium" sheet, line "Rebound effects / decreased immunity development."
- 109
"The most common reaction seen with SMC drugs during the pilot studies and during the first year of ACCESS-SMC was regurgitating the drug after ingestion. This was due to the unpleasant and lasting bitter taste of amodiaquine and because the hard tablets were not always crushed into a fine powder before mixing with water, which often caused children to gag and expel the medicine. Since the introduction of the orange flavored dispersible amodiaquine and SP tablets, this is no longer a common problem." Malaria Consortium, comments on a draft of this page, October 2017
- 110
- We have seen one estimate from Malaria Consortium that suggested that roughly 0.5%-1.5% of children who received SMC drugs vomited. See Malaria Consortium, 2013 operational data. We have not vetted this estimate. We have not seen other information about vomiting in Malaria Consortium's monitoring.
- Malaria Consortium, comments on a draft of this page, October 2017
- 111
"What should the Role Model Caregiver do if a child vomits during the observation period after giving a dose of SMC medicines? a) Refer the child to the health facility, b) Give the child water, c) Give the child another dose of each SMC medicine, [Correct answer: C]" Malaria Consortium Quiz Answer Key.
- 112
"It is very rare that children become sick after taking SMC medicines, but some children may feel a bit sick for a short while; what are some symptoms children may have? a) diarrhoea, b) itching, c) headache, d) mild abdominal pain, e) rash, f) a,b, and c, g) d,e, and f, h) All of the above, [Correct answer: H.]" Malaria Consortium Quiz Answer Key.
- 113
"In Nigeria, there was a follow-up of a cohort of 10,000 children one week after each SMC cycle to ask about side effects, and to assess the frequency of mild and moderate side effects that may not be reported to the health facility. […] The 10,000 children cohort in Nigeria produced only five PV reports, and it seems to be linked to some reluctance to report by beneficiaries." ACCESS-SMC, Progress update - Safety monitoring, pgs. 7 and 10.
- 114
- ACCESS-SMC, Reported severe adverse events.
- @Ndiaye et al. 2016@.
- ACCESS-SMC, Progress update - Safety monitoring
- 115
See the most recent version of the model here, "Malaria Consortium" sheet, line "Serious adverse events due to side effects of treatment."
- 116
- "We are only permitted to procure products from suppliers that meet WHO stringent guidelines for pre-qualification of malaria drugs to ensure high-quality and efficacy of SP and AQ." Malaria Consortium, comments on a draft of this page, October 2017
- "...there is not yet any other supplier who manufactures both products and packages them in a 1SP 3AQ treatment slide combination, irrespective of WHO guidelines. Unpackaged products could be used but it is significantly more inefficient with a much higher risk of error." Malaria Consortium, comments on a draft of this page, October 2019
- 117
Malaria Consortium, comments on a draft of this page, October 2020.
- 118
See our most recent model, "SMC" and "Results" sheets.
- 119
Additional funding will support work in 2022-23 because Malaria Consortium's work in 2021 will be fully supported by available funding. See this spreadsheet, sheet "RFMF projections," cell B9.
- 120
For a discussion of why we consider funding a charity's work up to three years in the future, see this blog post.
- 121
Specifically, we exclude the portion of Malaria Consortium's SMC portfolio that is funded by the Global Fund to Fight AIDS, Tuberculosis, and Malaria.
- 122
Some of our top charities have a policy of holding funding reserves. In our room for more funding analyses, we include reserved funding as funding available to support program activities. We do this both to ensure consistency across top charities (as not all top charities hold reserves) and to understand the true effect of granting additional funding (i.e. whether additional funding would support undertaking additional program activities versus building or maintaining reserves).
- 123
Open Philanthropy, a philanthropic organization with which we work closely, is the largest single funder of our top charities. The vast majority of Open Philanthropy's current giving comes from Good Ventures. We make recommendations to Open Philanthropy each year for how much funding to provide to our top charities and how to allocate that funding among them.
- 124
In our projections of future funding, we typically count only one year of funding that an organization receives as a result of being on our list of top charities in order to retain the flexibility to change our recommendations in future years.
- 125
Malaria Consortium is piloting SMC programs in Mozambique and Uganda in 2020-21. We have excluded the potential costs of scaling SMC within these countries and possibly expanding to a third country in 2021-23 from this analysis. We expect to learn more about these opportunities after completion of the pilot programs.
- 126
At the time of this writing, in late 2020, our understanding is that Malaria Consortium's scale for 2021 has largely been decided, so we take the 2021 scale as a given.
- 127
- In April 2014-March 2015, it appears that about 11% of Malaria Consortium's total expenditure was unrestricted. See "Resources expended" from 2015 and compare "Restricted funds" and "Total funds" columns on Malaria Consortium, Trustees' report and financial statements 2015, Pg 17. Percentage of spending coming from restricted funds calculation: 50,626,000 British pounds / 56,809,000 British pounds = 0.89.
- In April 2015-March 2016, it appears that about 14% of Malaria Consortium's total expenditure was unrestricted. See Malaria Consortium, Trustees' report and financial statements 2016, Pg 16. (Math: On the line "Total Expenditure", 5886/41189 = 0.143.)
- In April 2016-March 2017, it appears that 12% of Malaria Consortium's total expenditure was unrestricted. See Malaria Consortium, Trustees' report and financial statements 2017. (Math: On the line "Total Expenditure", 5,900/50,840 = 0.116.)
- Malaria Consortium told us that it often uses unrestricted funding to support overhead that is needed for some of the restricted grants it receives (sometimes funders' requirements do not allow it to spend on overhead), so these unrestricted funds are not available to support new programs and activities. GiveWell's non-verbatim summary of conversations with Malaria Consortium staff, November 7 and November 9, 2016.
- For a more detailed breakdown of how Malaria Consortium has spent unrestricted funds in the past, see Malaria Consortium, restricted and unrestricted expenditure analysis 2016. It seems possible that some of the funds spent on "Monitoring & surveillance of malaria" and "Malaria strategy support" supported aspects of SMC programs, but we are uncertain.
- Malaria Consortium told us that it agrees with the U.K. Department for International Development about how it will use Programme Partnership Agreement (PPA) funds. It told us that it decides which programs to apply for PPA funds with depending on what it believes will be most impactful, and then if it receives PPA funds it is committed to spending the funds on those programs. GiveWell's non-verbatim summary of conversations with Malaria Consortium staff, November 7 and November 9, 2016.
- Malaria Consortium told us that it does not expect to receive PPA funds beyond 2018 due to the discontinuation of this funding stream for all recipients by the UK government. GiveWell's non-verbatim summary of conversations with Malaria Consortium staff, November 7 and November 9, 2016.
- In 2018, Malaria Consortium told us that it has not allocated significant amount of unrestricted funding to either SMC programs or other program activities in the last year. Diego Moroso, Malaria Consortium Global Programme Director - SMC, email to GiveWell, July 31, 2018.
- Malaria Consortium told us in July 2019 that it did not have sufficient unrestricted funding from the prior year to allocate unrestricted funding to SMC (Christian Rassi, Malaria Consortium SMC Programme Director, email to GiveWell, July 31, 2019). It did allocate at least $100,000 of unrestricted funding to projects other than SMC (Madeleine Marasciulo, Malaria Consortium US Development Lead, conversation with GiveWell, March 4, 2019). This funding was from Malaria Consortium's US entity. Our guess is that a large portion of this funding was from donors who read our recommendation of Malaria Consortium's SMC program and gave to Malaria Consortium unrestricted, either intentionally or unintentionally. This is our guess based on Malaria Consortium noting (Madeleine Marasciulo, Malaria Consortium US Development Lead, email to GiveWell, February 22, 2019) that it has seen its unrestricted donations to its US entity increase from a very low level since GiveWell began recommending Malaria Consortium's SMC program as a top charity.
- 128
GiveWell SMC funding gaps analysis (April 2018), "Coverage by country" sheet, "Donor mapping" section.
- 129
This understanding is from this spreadsheet, sheet "Source: 2020-23 Projections," "Assumptions" section and from multiple conversations with Malaria Consortium.
- 130
- 131
Countries included: Benin, Burkina Faso, Cameroon, Chad, The Gambia, Ghana, Guinea, Guinea-Bissau, Mali, Niger, Nigeria, and Togo.
- 132
Countries not included: Mauritania and Senegal.
- 133
The funding gap is calculated by multiplying the number of eligible children by $4.96, Malaria Consortium's projected average cost per targeted child in 2021-2023. See this spreadsheet, sheet "Maintenance," line "All countries, 2021-2023."
- 134
Malaria Consortium, Donor landscape 2019, sheet "SMC Donor Landscape." 9.1 million is the total for 2019 in the source, less 0.7 million in Senegal, which we exclude from the analysis because we do not have an estimate of the size of the gap in Senegal in 2020.
- 135
Malaria Consortium, Donor landscape 2019, "Nigeria" sheet.
- 136
"Nigeria will see the largest increase 2019-20, thanks to increased Global Fund support and expansion to new states using philanthropic funding." Malaria Consortium, SMC donor landscape, 2020 (redacted).
The addition of PMI is from Malaria Consortium, comments on a draft of this page, October 2020.
- 137
In 2020:
- Burkina Faso: 1.6 million children out of about 3.9 million eligible children
- Chad: 1 million children out of about 1.9 million eligible children
- Nigeria: 3.9 million children out of about 11.9 million eligible children
All figures for children supported by Malaria Consortium's programs are from this spreadsheet, sheet "Source: 2020-23 Projections." All figures for total eligible children are from Malaria Consortium, SMC donor landscape, 2020 (redacted).
- 138
From Malaria Consortium, Answers to GiveWell's questions, July 2020 (unpublished):
- Burkina Faso: "It is likely that Global Fund will maintain the level of support provided in 2019 and continue to take on 10 additional districts. This is arrangement is likely to hold over the period 2021-23 and is the basis of the room-for-funding projections."
- Chad: "In Chad, there are indications that Global Fund will increase funding for SMC as of next year, but the scale of their support has not yet been confirmed. For the room-for-funding projections, we have taken this into account by reducing the expected philanthropically supported scale-up to two districts in 2021 and potentially another two in 2022, based on a recent request from the national malaria programme."
- Nigeria: "It is very likely that Global Fund will maintain the level of funding for SMC reached in 2020 (3 states, 5.5m children) for the period 2021-23, as well as take on one of the states currently supported philanthropically (1.3m children) and two to three states that are likely to be considered eligible as of 2021. This scenario has been factored into the budget projections in the room-for-funding analysis."
- 139
See this spreadsheet, sheet "Source: 2020-23 Projections," "Assumptions" section.
