In a nutshell
This page discusses voluntary medical male circumcision (VMMC) to curb the transmission of human immunodeficiency virus (HIV) and high-risk human papillomavirus (HPV), the cause of almost all cervical cancer.
There is strong evidence that VMMC significantly reduces the risk that men will acquire HIV: a meta-analysis of three high-quality randomized controlled trials (RCTs) found that circumcised men were roughly 50% less likely to acquire HIV in the first ~two years post-surgery.
There is also moderate quality evidence that VMMC reduces the risk that female partners of men will acquire high-risk HPV: the only RCT on the topic found a risk reduction of approximately 30% per year. Because high-risk HPV is the cause of almost all cervical cancer, we expect that VMMC would reduce the risk that female partners will develop cervical cancer. However, for reasons detailed in the report, we find it difficult to precisely estimate the conversion rate from high-risk HPV infections avoided to cervical cancer events avoided.
VMMC has some negative and offsetting impacts, among which are adverse events from surgery and the risk that men will engage in riskier sexual behavior following surgery. Based on the evidence collected so far, these impacts seem fairly minor relative to VMMC's positive effects. In the three RCTs we rely on, there was approximately one moderate or serious adverse event (e.g., infection, problems with appearance) for every 30 surgeries. Further, the trial evidence indicates that behavioral risk compensation does not pose a major risk to VMMC's effectiveness, though we would hope to see charities communicating carefully and clearly that VMMC is only partially protective against HIV infection.
Taking both HIV infections and cervical cancer events avoided into account, VMMC appears to be below the range of cost-effectiveness of programs we would consider directing funding to.
It is not clear to us at this stage whether the bottleneck to more voluntary circumcisions being performed in HIV-endemic countries is demand for circumcision, lack of funding for surgeries, or some other factor. Our light review of the funding landscape suggests it is possible that funding for surgeries may be the bottleneck in some countries where charities provide VMMC services.
Published: August 2016; Last updated: March 2021 (2016 version)
Table of Contents
- What are human immunodeficiency virus (HIV) and cervical cancer (the diseases targeted by VMMC)?
- What is VMMC and how does it curb HIV and high-risk HPV transmissions?
- What is the evidence regarding the general effectiveness of VMMC in curbing HIV transmission?
- What is the evidence regarding the general effectiveness of VMMC in curbing cervical cancer incidence?
- Other negative and offsetting impacts
-
How cost-effective is VMMC?
- Introduction and bottom line
- Assumptions and choices
- Variables excluded from the models
- Other cost-effectiveness analyses
- Information about HIV to inform how to weigh the value of avoiding an infection versus saving a life or other benefits
- Information about cervical cancer to inform how to weigh the value of avoiding events versus saving a life
- Global room for more funding for VMMC
- Our process
- Questions for a charity
- Sources
What are human immunodeficiency virus (HIV) and cervical cancer (the diseases targeted by VMMC)?
This report focuses on the protective effect that VMMC has in relation to HIV and cervical cancer. There is also randomized evidence that suggests that VMMC may have a protective effect in relation to herpes simplex virus type 2,1 trichomonas vaginalis,2 and HPV in men (which may sometimes lead to cancers of the penis, anus, and oropharynx (back of the throat)).3 However, we have not carefully reviewed that evidence. Rather, we focus this report on HIV and cervical cancer because they are the most important outcomes in global burden of disease terms.4
HIV and cervical cancer are a significant burden in Sub-Saharan Africa, where the World Health Organization (WHO) is prioritizing VMMC scale-up in fourteen countries. In priority countries, the WHO estimates that the average HIV prevalence rate among adults aged 15-49 is ~8.1%5 and the average percentages of total disability-adjusted life years (DALYs) and deaths attributable to HIV/AIDS are ~18.9% and ~20.2% respectively.6 In Sub-Saharan Africa, it is estimated that ~3.4% of women develop cervical cancer before age 75 and that it is the cause of death for ~2.6% of women.7 We have not vetted the estimates of the burden of HIV or cervical cancer.
HIV is a virus that kills or damages the body's immune system cells, specifically the CD4 cells (T cells).8 There is no cure for HIV, but the virus can be controlled using antiretroviral therapy (ART).9 HIV is spread by the transmission of certain bodily fluids, most often through unprotected sex with an infected person.10
Cervical cancer begins in the cervix.11 Almost all cervical cancers are caused by human papillomavirus (HPV), a common sexually transmitted virus.12 The International Agency for Research on Cancer has found that there are thirteen cancer-causing ("high-risk") HPV types.13 Our understanding is that the body's immune system will usually rid itself of a HPV infection within two years.14 However, persistent infections can ultimately cause cancer. According to one estimate, about 10% of women with high-risk HPV on their cervix develop persistent infections.15 The WHO estimates that it takes 15 to 20 years for cervical cancer to develop in women with normal immune systems but only 5 to 10 years in women with weakened immune systems, such as those with untreated HIV infections.16 We have not vetted this estimate.
What is VMMC and how does it curb HIV and high-risk HPV transmissions?
VMMC is the voluntary removal of the prepuce (foreskin) of the penis.17 There are three surgical techniques for VMMC: the forceps-guided method, the dorsal slit method, and the sleeve resection method, each of which requires local anaesthesia.18 In addition, the WHO has pre-qualified two devices for VMMC,19 which some experts argue might help to accelerate the scale-up of VMMC since devices may be less expensive, safer, and easier to distribute in resource-poor settings than surgery.20 However, the key randomized evidence on VMMC discussed below tested the protective effect of surgical VMMC administered alongside HIV prevention counselling and, in some cases, testing for HIV and other STIs. Further, a light review of VMMC charities' websites suggested to us that charities are not yet using devices. We therefore focus this report on surgical VMMC, but we would return to the question of whether VMMC devices are effective if a charity's programming included them.
Since 2007, the WHO and the Joint United Nations Programme on HIV/AIDS (UNAIDS) has recommended VMMC for countries with high HIV prevalence, generalized heterosexual epidemics, and low levels of male circumcision.21 Fourteen countries in Sub-Saharan Africa are prioritizing VMMC scale-up to 'catch up' uncircumcised men aged 15 to 49: Botswana, Ethiopia, Kenya, Lesotho, Malawi, Mozambique, Namibia, Rwanda, South Africa, Swaziland, Tanzania, Uganda, Zambia, and Zimbabwe.22 Our report and cost-effectiveness analysis focus on these priority countries because we expect that they may have the most promising giving opportunities.
We have not thoroughly researched the biological mechanism by which VMMC reduces HIV acquisition. One review theorizes that it has a protective effect for HIV-negative men because it removes a major portal of HIV entry, the inner mucosal lining of the foreskin.23 We have not investigated this theory.
What is the evidence regarding the general effectiveness of VMMC in curbing HIV transmission?
There is strong evidence from three large randomized controlled trials that VMMC has a substantial protective effect for HIV-negative men (in the range of a roughly 50% reduction in the risk of acquiring HIV during the first ~2 years post-surgery). VMMC does not appear to reduce the risk of HIV-negative women acquiring the virus from HIV-positive partners. We discuss some potential limitations of the core studies underlying the protective effect of VMMC below. We have not thoroughly investigated all of these potential limitations but do not currently see them as major threats to the view that VMMC has a substantial protective effect against HIV.
Observational evidence on which we do not put much weight suggests there is no protective effect for the sub-group of men who have sex with men but potentially some effect for men within that group who have an insertive role in intercourse.
Transmission to HIV-negative males
The best evidence for VMMC's effectiveness comes from three randomized controlled trials (RCTs) with large sample sizes that found similar results. These trials are reviewed in Siegfried et al 2009, a Cochrane review and meta-analysis. Their combined results showed an incident risk ratio of 0.46 (i.e., a 54% reduction in the risk of acquiring HIV) at 21 or 24 months following circumcision (95% CI: 0.34 to 0.62).24
We have separately found, examined, and summarized these papers, which examined the protective effect of VMMC to curb acquisition of HIV by men in South Africa, Kenya, and Uganda.25
Table 1: Summary of results from 3 RCTs evaluating the effect of VMMC on male HIV acquisition
| Study | Sample size | Location | Incidence rate in control group | Incidence rate in treatment group | Follow-up (months) | Treatment effect (% protective effect) |
|---|---|---|---|---|---|---|
| Auvert et al 2005 | 327426 | Orange Farm, South Africa27 | 2.1 per 100 person years28 | 0.85 per 100 person years29 | 18.1 (mean of IQ range)30 | 60% (95% CI: 32%-76%)31 |
| Bailey et al 2007 | 278432 | Kisumu District, Kenya33 | 4.2% (over two years)34 | 2.1% (over two years)35 | 24 (median)36 | 53% (95%CI: 22%-72%)37 |
| Gray et al 2007 | 499638 | Rakai District, Uganda39 | 1.33 per 100 person years40 | 0.66 per 100 person years41 | 2442 | 51% (95% CI: 16%-72%)43 |
Study characteristics:
- Study population. The South Africa and Kenya trials enrolled 18-24 year-old uncircumcised participants. The Kenyan trial, but not the South African trial, required HIV-negative status for enrollment.44 The Uganda trial enrolled 15-49 year-old, HIV-negative, uncircumcised participants.45
- Blinding. Auvert et al 2005 blinded outcome assessors, counsellors (unless circumcision status was divulged), and personnel in charge of testing and collecting results.46 Bailey et al 2007 blinded the nurse-counsellors who administered testing and counselling (unless circumcision status was divulged). However it is not clear whether outcome assessors were blinded.47 The methods of blinding in Gray et al 2007 are not reported.48 Study participants were not blinded to their treatment status due to the nature of the intervention.
- Allocation concealment. Auvert et al 2005 and Gray et al 2007 did not ensure that the envelopes used to randomly allocate trial participants into the treatment and control arms were opened one at a time and in order, introducing some risk of bias.49 Bailey et al 2007's method of concealing the allocation of participants is not completely reported.50
- Implementation method. In Auvert et al 2005, circumcisions were performed by local general practitioners experienced with VMMC, in their surgical offices, using the forceps-guided method.51 No post-operative appointments were scheduled. In Gray et al 2007, circumcisions were performed by "trained and certified physicians in well-equipped operating theatres" using the sleeve procedure, with 3 post-operative appointments scheduled.52 Finally, in Bailey et al 2007 surgeries were performed at the study clinic, with study clinicians, using the forceps-guided method, again with three post-operative visits scheduled.53 In all the studies, participants received counselling and testing at all trial related follow-ups.54 HIV status was confirmed via blood tests.55
- Compensation. In Auvert et al 2005, participants received 300 South African rand as compensation.56 In Gray et al 2007, participants received US$5 at screening and enrollment, $5 at surgery, and $5 at completion of post-operative follow-up. Compensation for routine follow-up visits was $3.57 Finally, in Bailey et al 2007, participants received 300 Kenyan shillings for each study visit.58
- Analysis. In all 3 RCTs, data were analyzed based on initial treatment assignment (i.e., an intention-to-treat analysis).59 In Auvert et al 2005, in the intervention group, 6.5% (93/1432) of participants were not circumcised at month three and in the control group, 10.3% (114/1105) were circumcised at month 21 (the final follow-up).60 Gray et al 2007 reported that 5.9% (146/2474) of the intervention group did not receive surgery within 6 months of enrollment in the study and that 1.3% (33/2522) of the control group was circumcised over the course of the study.61 Bailey et al 2007 reported that 0.7% (9/1277) of the intervention group were not circumcised at the first-month follow-up visit and that 1.6% (12/744) of the control group had been circumcised at the 24-month visit.62
We note some potential limitations of these studies:
- Trial stoppage. All trials were stopped early due to significant findings at the interim stage, consistent with pre-determined early stopping rules that (according to Siegfried et al 2009) are considered acceptable statistical practice.63 Early stoppage introduced significant additional attrition into each trial, though all trials used methods to try to reduce the potential bias introduced by such attrition.64 We have not carefully reviewed or vetted the methods used to reduce this bias. There is a risk that early stoppage led to the magnitude of the protective effect of VMMC being overestimated.65 The fact that the magnitude of the effect was fairly consistent across the three trials may partially mitigate this concern.66 We have not carefully reviewed the literature on early stoppage of trials. It is possible that reviewing this literature would allow us to better predict how much the treatment effect may be overestimated due to early stoppage.
- Loss to follow-up. Siegfried et al 2009 rate the risk of bias from incomplete outcome reporting from loss to follow-up as moderate in all three trials.67 Attrition rates (both because of early stoppage of the trial and because of general loss to follow-up) were high, but they were roughly equal between the treatment and control groups, and therefore we do not see strong reason to be concerned that they would significantly bias the estimated effects.68
- Limits to generalizability. The confidence we would have in a charity replicating the trials' protective effect would depend somewhat on that charity implementing all elements of the program studied, including counselling. Though there may be some self-selection bias in the trials, in that participants were those in the population connected enough to know of the studies and willing to enroll, the large sample sizes in all the trials mitigate this concern; we do not see strong reason to believe that the populations studied in these RCTs are relevantly different from populations who would receive VMMC in similar contexts.
- Blinding and allocation concealment measures were imperfect. As noted above, methods for blinding and allocation concealment were either imperfect or not fully reported. This introduces a risk of bias in either the selection of or the treatment afforded the treatment group vis-à-vis the control group. Rather than carefully investigating these potential biases ourselves, we defer to Siegfried et al 2009 on the importance of these biases to the validity of the studies’ findings; Siegfried et al 2009 concludes: “Reporting of methodological quality was variable across the three trials, but overall, the potential for significant biases affecting the trial results was judged to be low to moderate given the large sample sizes of the trials, the balance of possible confounding variables across randomised groups at baseline in all three trials, and the employment of acceptable statistical early stopping rules.”69
There are post-trial follow-up studies for two of the above RCTs:
- Mehta et al 2013 (a follow-up to Bailey et al 2007) compares a non-randomized subset of uncircumcised and circumcised participants from the Kenya trial for about 6 years post-surgery, finding a sustained risk reduction of 58% (95% CI: 34% to 74%).70 Due to the non-randomized study design, we do not put weight on Mehta et al 2013's findings when predicting VMMC's long term effects.
- Gray et al 2012 (a follow-up to Gray et al 2007) maintained surveillance of Uganda trial participants for up to 4.79 years.71 Members of the control group were offered circumcision at the conclusion of the initial two-year study period, and this follow-up study compares circumcised men from both treatment arms to men from the control group who chose to remain uncircumcised. Our view is that this methodology creates significant potential for bias, and for that reason we do not put weight on this study.72
The fact that there is no published follow-up study to the first RCT on VMMC (Auvert et al 2005) suggests to us some risk that follow-up data was collected but not published because the results were unfavorable. However, a non-randomized follow-up study comparing HIV incidence between circumcised and uncircumcised men in the South African township of Orange Farm (where the RCT was conducted) found an estimated reduction in HIV incidence rate for circumcised men when compared to uncircumcised men of 57% - 61%.73 These results somewhat mitigate our concern about publication bias.
Transmission between men who have sex with men
We found no RCT testing the hypothesis that VMMC curbs HIV acquisition among men who have sex with men. A 2011 Cochrane systematic review on this topic found 21 observational studies, the pooled effect estimate of which was not statistically significant or homogeneous (20 studies; n = 65,784).74 There was a statistically significant pooled effect estimate for the subset of studies examining men who reported an insertive role during intercourse (7 studies; n=3465), estimating a 73% risk reduction (OR 0.27, 95% CI 0.17-0.44).75
However, as we have said in previous posts about our principles for assessing evidence, we do not place much weight on observational data like these because of the difficulty of attributing the reported effect to the studied intervention.
HIV-positive male to HIV-negative female transmission
The best available evidence indicates that VMMC does not provide protection for HIV-negative female partners of HIV-positive circumcised men. There is one RCT on this topic, conducted in Rakai District, Uganda. Wawer et al 2009 randomized uncircumcised, HIV-infected, asymptomatic men into circumcision and control groups, enrolled the HIV uninfected female partners of these men (circumcision, n=93; control, n=70), and followed up at 6, 12, and 24 months.76 The trial was stopped early for futility because the 24 month results showed no risk reduction for HIV acquisition in the studied women.77
What is the evidence regarding the general effectiveness of VMMC in curbing cervical cancer incidence?
Introduction and bottom line
Persistent high-risk HPV infections cause almost all cases of cervical cancer.78 There is evidence from one RCT that VMMC reduces the incidence of high-risk HPV in the HIV-negative female partners of circumcised men by approximately 23% and increases the likelihood that those partners will clear a high-risk HPV infection within a year by approximately 10% (details and citations below). These effects were observed over a two-year period. Accordingly, we expect that VMMC reduces the risk of cervical cancer in female partners by reducing high-risk HPV.
We describe some limitations of the evidence below. In general, we think that the evidence on cervical cancer is less convincing than the link between HIV acquisition and VMMC. This is because there is only one RCT, that RCT has less statistical power due to its smaller sample size (n=1245; compared to sample sizes of between 2784 and 4996 in the HIV trials), and the trial was conducted in a less generalizable population (only women in spousal or long-term partnerships). Nevertheless, our best guess is that VMMC reduces the risk of acquiring cervical cancer.
There is no direct RCT evidence on the magnitude of VMMC's protective effect for cervical cancer in female partners. It is difficult to estimate the conversion rate of averted high-risk HPV infections to averted cervical cancer events. That ratio will depend on the available facilities for screening and treatment of pre-cancerous lesions, prevalence of HIV, and secondary effects from VMMC decreasing the overall numbers of HPV and HIV infections. Our cost-effectiveness analysis below therefore offers only a very crude analysis of the conversion rate from high-risk HPV to cervical cancer.
We have not thoroughly researched the biological mechanism(s) underlying this evidence, but some experts argue that VMMC reduces penile HPV carriage,79 which seems plausible to us because it accords with two randomized studies of high-risk HPV prevalence in men which indicate that VMMC reduced the prevalence of high-risk HPV in circumcised men by ~34% over ~two years.80 We have not thoroughly vetted these studies, but they give us slightly more confidence in VMMC’s protective effect against women acquiring high-risk HPV.
Evidence that VMMC curbs high-risk HPV
We rely primarily on Wawer et al 2011, an RCT in Rakai District, Uganda which tests the hypothesis that VMMC reduces the risk that a man's female partners will acquire high-risk HPV.81 The study's core methods and results are:
- Results. Over the 24-month trial period, partners of circumcised men were 23% (95% CI: 7%-37%) less likely to acquire high-risk HPV in a given year than partners of uncircumcised men.82 In addition, high-risk HPV clearance was 10% more likely in women in the intervention group than in the control group (95% CI: 3%–20%).83
- Outcomes. The authors defined 'acquisition' of high-risk HPV as the detection of one or more new high-risk HPV genotypes within a one-year follow-up interval, even where a woman was already infected with a different genotype.84 They defined ‘clearance' of high-risk HPV as the proportion of pre-existing high-risk HPV genotype-specific infections that were negative for that genotype at a subsequent study visit.85
- Sample. Trial participants were recruited via the men randomized in both Ugandan studies of VMMC discussed above (Gray et al 2007 and Wawer et al 2009).86 Men who were married or in long-term consensual relationships were asked to identify their female partners, who in turn were invited to enroll in this trial.87 To avoid bias to HPV results, HIV-positive people were not enrolled, and women were excluded in the analysis phase if they or their partners acquired HIV in the trial period. The authors wanted to avoid potential bias introduced by the fact that HIV increases the risk of acquiring high-risk HPV and that more people acquired HIV in the control than the treatment group.88 Further, analysis only included women who enrolled contemporaneously with their partners.89 648 female partners of men randomized to undergo VMMC, and 597 female partners of men randomized into the control group enrolled.90 Participants were offered free HIV testing and prevention counselling.91
- Randomization. Since the enrollment of female partners in the trial was voluntary, it seems possible that there could be non-random differences in partners’ decisions to enroll between the control and treatment groups. However, we do not see any reason to believe that this is the case. Only women who enrolled before their partner's allocation was known are included in the sample,92 and the intervention and control groups are roughly comparable on observable factors (age, marital status, religion, education, HPV status) and reported behavioral factors (e.g., number of sexual partners in the past year) at baseline.93
Limitations to using Wawer et al 2011's results to think about VMMC's protective effect for cervical cancer include:
- Proxy outcome. The study examines a risk factor (high-risk HPV infection) which is several steps removed from the clinical end point of interest to us (cervical cancer). It is therefore difficult to use it to formulate an estimate of VMMC's protective effect for cervical cancer, especially given that such an estimate would need to take account of both the reduced risk that female partners of circumcised men will acquire high-risk HPV and their increased chance of clearing any infection they do acquire. Further, clearance of HPV-16, one of the strains most strongly associated with cervical cancer,94 was actually statistically significantly lower in the intervention group in the trial.95 However, a cervical cancer expert whom we spoke with on a confidential basis told us that there is no biological reason to believe that VMMC would be more protective against some strains of HPV than others; rather, they would guess that the different observed relative risk reductions were due to randomness and that the actual risk reduction from VMMC is similar for all strains of high-risk HPV.
- Generalizability. The study population is limited to spousal or long-term partnerships where both parties are HIV-negative.96 Sexual activity and risk-taking behaviors may be more diverse in the general population than in the subset of people in spousal or long-term relationships. It is difficult to confidently predict the extent to which these differences will affect VMMC’s impact on the spread of high-risk HPV, but VMMC may have a smaller protective effect for the average woman than it does for the sub-population of women in monogamous partnerships. On the other hand, the study likely underestimates VMMC's protective effect for cervical cancer in relation to the sub-group of HIV-positive women because people with HIV are more likely to develop a persistent high-risk HPV infection.97
- Exclusion of men and women who acquired HIV. The analysis excludes both women who acquired HIV during the trial and women whose partners acquired HIV during the trial.98 More men in the control arm acquired HIV.99 Excluding them made the treatment and control groups unbalanced, putting the study at significant risk of bias and reducing our confidence in the results. In particular, the decision to exclude participants who acquired or whose partners acquired HIV excludes more people whose risky sexual behaviors likely render them more liable to HPV infection from the control arm than from the intervention arm. Thus, the study likely underestimates VMMC's protective effect for high-risk HPV, though imbalance between the treatment and control groups reduces our confidence in the study’s results overall.
- Attrition. Attrition rates over the trial were high -- approximately 17% -- however they were roughly equal between the treatment and control groups, and therefore we do not see strong reason to be concerned that they would significantly bias the estimated effects.100
Other negative and offsetting impacts
Adverse events
One drawback of VMMC is adverse events from circumcision surgery. No mortalities caused by surgery were recorded in the RCT evidence we rely on, but moderate or severe events (e.g., infection, problems with appearance) occurred in ~3.8% of surgeries performed.
Evidence
We rely on the adverse events reported in the South Africa and Uganda trials of VMMC, Auvert et al 2005 and Gray et al 2007. We do not put much weight on the rate of adverse events reported in the Kenya trial, Bailey et al 2007, because it was seen as unusually low: mild or moderate events occurred in only 1.5% of circumcisions performed.101 Indeed, Bailey et al write that VMMC implementers should not expect such a low adverse event rate because the quality of medical care the Kenya trial offered was very high.102
Auvert et al 2005's implementation methods are most akin to what we believe a charity might employ: experienced local practitioners performed the surgeries,103
and post-operative appointments were only provided when a patient presented with a complication.104
In this study, adverse events occurred in 3.8% of surgeries performed (60/1,568). A breakdown of the kinds of events appears here:105

Gray et al 2007 does not report whether surgery was performed by local staff or trial staff, simply that "trained and certified physicians" did the circumcisions and made three post-operative appointments.106 The authors reported adverse events at a higher level of generality than Auvert et al 2005, pre-classifying them as mild (no treatment required), moderate (treatment required) and severe (surgical intervention, hospitalization or referral for specialized care required).107 They recorded moderate adverse events in 3.4% of surgeries (79/2328) and severe adverse events in 0.2% of surgeries (5/2328), roughly consistent with the adverse event rate from Auvert et al 2005.108
Consequences for our view of a charity implementing VMMC
VMMC's adverse event rate may depend substantially on a charity's implementation method. Our impression is that adverse event rates for VMMC in Africa outside of trial settings are poorly documented, though one prospective study in Bungoma District, Kenya found a rate as high as 17.7%.109 We are aware of some evidence that VMMC can be performed safely by non-physician providers, or a team incorporating non-physician providers, and would expect to investigate this evidence further if a charity incorporated some delegation into its programming.110
Behavioral risk compensation
VMMC is only partially protective.111 There exists a concern that circumcised men may feel protected against HIV and engage in riskier sexual behavior that dilutes or erases VMMC's protective effect (“behavioral risk compensation”). In all the RCTs discussed above, self-reported data indicated that risky sexual behaviors were higher in the intervention group than in the control group over the trial period (details and citations below). However, that difference was statistically significant on only a few variables, and there remained a large protective effect of VMMC at 24 months. Research conducted in a programmatic setting in Kenya comparing circumcised men with matched controls found no increase in behavioral risk compensation in the 24 months following surgery.112 However, it is noteworthy that all data about behavioral risk compensation is self-reported and may therefore underestimate risky sexual behavior.
We do not see reason to believe that behavioral risk compensation poses a major risk to VMMC's effectiveness for men because the effect size estimates in the RCTs discussed above incorporate any offsetting effects of behavioral risk compensation. However, if the self-reported data on behavioral risk compensation is inaccurate and circumcised men are engaging in riskier sex, then this may result in increased HIV risk for women. Overall, based on the evidence collected so far, we do not see behavioral risk compensation as a major concern. However, we would hope to see charities communicating carefully and accurately about the protective effect of VMMC to minimize the potential for behavioral risk compensation in populations with whom they work.
The RCT in South Africa (Auvert et al 2005) measured behavioral disinhibition using self-reported data about marital status, number of non-spousal partners, sexual activity, number of sexual partners, and attendance at a clinic for a health problem related to the genitals.113 All of these factors were higher in the first year of follow-up for the intervention group, and four out of five were higher during the second year.114 However, only the mean number of sexual contacts was statistically significantly higher in the intervention group (7.5 versus 6.4 at 21 months).115 The Kenya and Uganda RCTs of VMMC measured behavioral disinhibition using self-reported data about number of sexual partners, extra-marital sex/sex with someone other than one's regular partner, condom use, alcohol use with sexual intercourse, and transactional sexual intercourse.116 In the Uganda trial, there was no variable on which the intervention group had statistically significantly higher self-reported risk-taking behaviors than the control group.117 In the Kenya trial, circumcised men exhibited slightly riskier behavior on all five assessed measures at month 24, and this was significant for two of the measures: unprotected sexual intercourse with any partner in the previous 6 months and consistent condom use.118 However, the authors argue that differences between intervention and control groups were attributable to increases in safer sexual practices in the control group rather than to riskier behavior patterns in the circumcision group, indicating that risk compensation may not have occurred during the trial period.119 We have not vetted this claim.
Reduced sexual pleasure
Though some scientists argue that circumcision results in reduced sexual pleasure for men,120 our impression is that the link between circumcision and reduced sexual pleasure remains disputed in the literature.121 We have not examined this literature closely because a) we would expect the protective effects of VMMC in priority country contexts to substantially outweigh potential effects of reduced sexual pleasure for those electing to access VMMC, and b) we expect that it would be challenging for us to form a reliable view of the relevant literature in a relatively short amount of time.
Consent to treatment
The final negative and offsetting impact of VMMC may be difficulties ensuring that consent to be circumcised is full and informed. For example, it is an open question whether adolescents are capable of giving full and informed consent for surgery that will affect their adult sexual lives. We have not thought much about these ethical issues but would expect to consider them more closely when assessing a VMMC charity.
How cost-effective is VMMC?
Introduction and bottom line
Based on a cost-effectiveness model we put together in August 2016 and updated with our most recent moral weights as of February 2021, we estimate that VMMC is below the range of cost-effectiveness of the opportunities that we expect to direct marginal donations to (about 10x cash or higher, as of 2021).122
Note that our cost-effectiveness analyses are simplified models that do not take into account a number of factors. There are limitations to this kind of cost-effectiveness analysis, and we believe that cost-effectiveness estimates such as these should not be taken literally due to the significant uncertainty around them. We provide these estimates (a) for comparative purposes and (b) because working on them helps us ensure that we are thinking through as many of the relevant issues as possible.
Our cost-effectiveness analysis incorporates five major costs and benefits: (1) HIV mortalities avoided, (2) HIV infections avoided (separated into persons who receive ART and those who do not), (3) cervical cancer events avoided, (4) cervical cancer mortalities avoided, and (5) adverse events caused by surgery.123 Our analysis is relatively simplified and unlikely to capture all of the key issues, especially in relation to cervical cancer.
The cost-effectiveness of VMMC varies substantially depending on incidence rates for HIV and cervical cancer in the areas where it is implemented. Download GiveWell’s VMMC cost-effectiveness estimate, country-by-country analysis for more information.124
Assumptions and choices
Our cost-effectiveness analysis relies on a variety of assumptions about which we are uncertain. Some important assumptions and choices made in our model for the number of HIV infections averted are:
- Choice of population data. Where possible, we have used mean population data (e.g., the cost per VMMC, the HIV incidence rate) from the countries where we expect giving opportunities to be situated and noted our sources in comments to the model. We have not vetted the data underlying them.125
- Cost of identifying candidates for VMMC. We include a rough premium for testing and counselling for men who try to access VMMC but test HIV-positive and therefore do not benefit from VMMC's protective effect in relation to our core outcomes.126
- Risk reduction rates. As our best guess for the magnitude of the protective effect of VMMC, we use the pooled risk reduction rates from the Siegfried et al 2009 Cochrane review to estimate VMMC's risk reduction rate in years 1 and 2 (0.5, 0.54) and the average of those rates (0.52) thereafter (the corresponding 95% confidence interval for these rates in Siegfried et al 2009 ranges from 0.28 to 0.66).127 There are a variety of reasons that using these risk reduction rates could lead to either a slight overestimate or underestimate of the cost-effectiveness of the intervention (details in the following footnote).128
- Decreasing HIV incidence. We expect that HIV interventions implemented contemporaneously with VMMC, such as the distribution of antiretroviral therapy, will also decrease the HIV incidence rate. To account for this effect, we decrease the HIV incidence rate by about 7% per year. This figure is the average per year decrease in HIV incidence in priority VMMC countries from 2011 to 2014, based on UNAIDS data. We have not investigated the accuracy of this data.129
- Population modelling. We monitor community level effects crudely. To model infections avoided in the cohort of men who undergo VMMC over time, we simply remove infected and deceased persons from the intervention population each year.130 We note that our model as presented does not account for the fact that someone who avoids an HIV infection one year may acquire HIV in a future year. However, we have informally tested the sensitivity of the model to this assumption, and it does not materially affect our bottom line. We also implicitly make a number of other assumptions about the population receiving VMMC in our model (details in following footnote).131
- Secondary effects. To capture secondary effects -- fewer infected men in turn leading to fewer infected women in turn leading to fewer infected men -- we apply a multiplier of 1.5 to the total number of infections avoided.132 We have not vetted the accuracy of this multiplier and encourage interested readers to experiment with other input values.
- Timing of negative effects of HIV. On average, acute physical suffering and death from HIV/AIDS occurs ~10 years after infection.133 Accordingly, our model discounts the benefits of VMMC back 10 years.134 However, our guess is that significant negative psychological, social, and economic impacts occur earlier – both at the time a person discovers their positive status and at the time it becomes known to others. (We discuss these issues below.) We therefore encourage interested readers to tailor the discount to their assessment of when the negative effects of HIV would accrue.
- Time horizon. We have largely arbitrarily chosen to count only about 20 years’ worth of benefits from VMMC in this cost-effectiveness analysis. A few reasons for this choice of time horizon are: a) we are highly uncertain about whether core assumptions underlying our model will apply multiple decades into the future; shorter time horizons limit the influence of far future effects on our model, b) a 20-year time horizon is roughly similar to time horizons we have used in our other cost-effectiveness analyses, which simplifies comparisons between our analyses, and c) it is our impression that using a 20-year time horizon is fairly common in the cost-effectiveness literature.
Some important assumptions and choices made in our model of cervical cancer events and mortality avoided are:
- Risk reduction rates. We have estimated the risk reduction that VMMC affords female partners of uncircumcised men for contracting high-risk HPV using data from Wawer et al 2011.135 Wawer et al 2011's data is drawn from a sample of HIV-negative women who met the criterion of being in a spousal or long-term relationship with an HIV-negative man. Sexual activity and risk-taking behaviors may be more diverse in the general population than in the subset of people in spousal or long-term relationships, and it is difficult to confidently predict the extent to which such differences may affect VMMC’s impact on the spread of high-risk HPV. As a very rough estimate, we apply a 30% discount to VMMC's protective effect for cervical cancer to account for the fact that some of the population are not monogamous. We have low confidence in this discount.136
- Cervical cancer incidence and mortality rates. We estimated cervical cancer incidence and mortality rates using Arbyn et al 2011's study of the worldwide burden of cervical cancer in 2008, specifically the study's data from East Africa, which is the region listed that is most relevant to priority countries.137 We have not vetted these data. We note the incidence and mortality rates may not be generalizable to priority countries in Southern Africa and may have changed over time. For example, Forman et al 2012 argue that global HPV incidence has been decreasing by 2% per year, which would lead to a lagged concomitant reduction in cervical cancer incidence.138
- Conversion rate from high-risk HPV to cervical cancer. We have extremely little confidence in how we are modeling conversion from high-risk HPV to cervical cancer. As a very rough approximation, we assume a linear relationship: our model assumes that a 30% relative risk reduction for high-risk HPV translates to an equivalent 30% relative risk reduction for cervical cancer. We spoke with a cervical cancer expert on a confidential basis who believed this is a reasonable if highly uncertain way to model the relationship. A further assumption arises because Wawer et al 2011 calculated high-risk HPV incidence by measuring whether a woman acquired any new high-risk genotypes within a year (its headline effect did not distinguish between women who acquired one new high-risk HPV genotype and those who acquired many; also, women who acquired a new high-risk HPV genotype may have already had other genotypes at baseline). So far as a risk reduction estimate for high-risk HPV calculated by this measure does not capture the decreased relative risk of developing cervical cancer in a linear way, our model is lacking. However, we could not easily find information to help us improve our modeling of the relationship between acquiring a new strain of high-risk HPV and developing cervical cancer; our impression is that there is not a "rule-of-thumb" conversion rate accepted by the medical community.139
- No population modeling. We do not attempt to do detailed population modeling (like we did for VMMC’s effect on HIV) to estimate the community-level effects of VMMC on cervical cancer. We also do not adjust for other detailed population-level factors such as the fact that HIV-infected persons are more likely to develop a persistent high-risk HPV infection.140 Because the model is very crude, we have not corrected for these limitations. We may improve this modeling if we decide to investigate this intervention further in the future.
- Discount for uncertainty about key effects in the model. We are very uncertain about many aspects of how we model VMMC’s effect on cervical cancer. We incorporate discounts to account for concerns around external validity, replicability, and uncertainty in how we have modeled the relationship between HPV and cervical cancer; together, these add to a ~50% discount on the benefits of VMMC for cervical cancer.141
Variables excluded from the models
We have excluded certain variables because we do not expect them to relevantly affect our overall view of the relative priority of VMMC and, in some cases, because they are difficult to properly quantify.
Excluded variables that may increase the cost-effectiveness of VMMC include:
- VMMC may lower the risk for men of acquiring HSV-2 (herpes) and high-risk HPV, which can cause penile, anal and oropharynx cancer.
- VMMC may lower the risk of female partners acquiring STIs such as trichomonas vaginalis.
- VMMC's wide range of protective effects suggests to us that current evidence may underestimate VMMC's overall protective effect for men and their partners.
- VMMC may reduce financial costs to governments and healthcare systems via its effect on HIV and other outcomes.
Excluded variables that may reduce the cost-effectiveness of VMMC include:
- VMMC may reduce the sensitivity of the penis and therefore sexual pleasure.
- VMMC may cause some men to engage in riskier sexual behavior.
- VMMC may cause alienation within certain cultures and religions.
Other cost-effectiveness analyses
We have only done a cursory review of other academic literature on the cost-effectiveness of VMMC.142 The estimates that we have seen in this literature for cost per HIV infection averted range from $181 to $4,096.143 We do not put much weight on these other estimates, especially since we have not carefully reviewed them.
Information about HIV to inform how to weigh the value of avoiding an infection versus saving a life or other benefits
Mortality. Our impression is that HIV-infected persons who access antiretroviral therapy (ART) before they have progressed to auto-immune deficiency syndrome (AIDS), the final stage of HIV infection, may have a near-normal life expectancy.144 Without treatment, however, we have seen various studies suggesting that cumulative mortality in Sub-Saharan Africa is ~50% in the ten years following HIV infection.145 Of these studies, we rely mainly on a prospective cohort study conducted in rural Uganda, because it has the longest follow-up period (13 years) and was conducted in a VMMC priority country.146 The cohort's survival curve was as follows:147

According to UNAIDS, roughly 69.5% of people in Sub-Saharan Africa that WHO clinical guidelines state should be on ART are untreated.148
Physical symptoms. Salomon et al 2015 (a publication of the Global Burden of Disease 2013 study) estimates that the DALY weight (0 being full health and 1 being equivalent to death) for living with symptomatic HIV before it develops into AIDS is about 0.27, for HIV/AIDS cases receiving ART is about 0.08, and for AIDS cases without ART is about 0.58.149 We have not carefully reviewed the methodology behind these estimates. The WHO estimates that without treatment, most HIV-positive people will develop signs of HIV-related illness (as the immune system weakens) within 5 to 10 years, though this period can be shorter, and that the time between acquiring HIV and AIDS is usually between 10–15 years, but sometimes longer.150 In Sub-Saharan Africa, we have seen estimates of median time between HIV infection and progression to AIDS (without treatment) ranging from 6.2 to 9.4 years.151 We have not vetted any of these estimates. AIDS allows opportunistic infections to cause severe, often fatal, illnesses.152 Other symptoms include recurring fever or profuse night sweats; extreme and unexplained tiredness; sores of the mouth, anus, or genitals; pneumonia; and memory loss.153
Negative psychological and social impacts of HIV-positive status. HIV-positive status in Sub-Saharan Africa may attract stigma and discrimination. Consequences may include: loss of livelihood (whether due to dismissal from employment, or fewer clients as the person begins to show symptoms of the disease), loss of marriage and childbearing options, poor care (including refusal of care) within the healthcare sector, withdrawal of care-giving by others in the home, loss of hope, and feelings of worthlessness.154 These impacts may accrue earlier than HIV's physical symptoms, either when the infection is discovered by testing or when it is disclosed to others.
Information about cervical cancer to inform how to weigh the value of avoiding events versus saving a life
Cervical cancer is preventable and curable if detected early. However, Sub-Saharan Africa has what the WHO describes as a "lack of effective screening and treatment policy,” meaning that “most women seek consultation only when the disease is already at an advanced stage."155 Accordingly, we have seen relevant estimates for cervical cancer's case-fatality rate between 57.8%156 and 76.5%.157 We have not vetted these estimates.
Common symptoms of advanced cervical cancer include weight loss, fatigue, back pain, bone fractures and leakage of urine or feces from the vagina.158 Little treatment is available. The WHO writes: "The limited resources available for treatment are not enough to provide effective surgical, radiotherapy and chemotherapeutic services. Not much of the palliative care needed at this stage of the disease is available."159 Cervical cancer also has a significant destabilizing effect on families, as the disease primarily affects young adult women.160
Global room for more funding for VMMC
It is not clear whether insufficient funding for surgeries, demand for VMMC, or some other factor is the bottleneck to more voluntary medical circumcisions being performed in VMMC priority countries.161
Our review of the funding landscape suggests it is at least possible that funding for surgeries rather than lack of demand for VMMC is precluding the scale-up of VMMC in some places.162 Further, a brief, informal website-based review of the current VMMC funding commitments of major organizations that have historically funded VMMC in priority countries indicates to us that there may be a funding gap for surgeries, though we are highly uncertain. Some of the major organizations that have funded VMMC in the past include the US President's Emergency Plan for AIDS Relief (PEPFAR), the Bill & Melinda Gates Foundation, Population Services International, the Maternal and Child Health Integrated Program (a USAID Division) and the Global Fund to fight AIDS, Tuberculosis and Malaria.163 One estimate we have seen is that an additional $710 million would be needed to achieve UNAIDS' goal of 80% circumcision coverage in priority countries by 2016.164 We have not vetted this claim.
Further, we have not reviewed the literature on interventions to increase demand for VMMC, but we have seen one RCT that tests the effect of providing a small amount of monetary compensation on circumcision rates, finding that it may increase VMMC uptake by ~4x.165
Our process
In forming our views on whether VMMC reduces the risk of HIV acquisition, we relied heavily on Siegfried et al 2009. We also searched for RCTs or quasi-RCTs related to VMMC published after 14 June 2007 (the final date of the Cochrane review166 ) by searching PubMed and Google Scholar, and tracing citations to resolve research questions. In addition, we looked at papers citing the three RCTs underlying Siegfried et al 2009 and conducted searches for "VMMC" and "cost" to inform our cost-effectiveness analysis. In forming our views on the connection between VMMC and high-risk HPV, we relied heavily on Wawer et al 2011.
To find clinical and prevalence information related to HIV/AIDS and cervical cancer, we employed Google Scholar and Google searches, relying on papers looking at overall burden of disease or information published from relatively reputable health bodies such as the Centers for Disease Control and Prevention, the World Health Organization, and UNAIDS. To consider room for more funding, we conducted Google searches aimed to identify the major funders and advocacy organizations working on VMMC and then reviewed their published materials.
Questions for a charity
While not an exhaustive list, we would ask the following questions of any charity applying for a recommendation based on its work on VMMC:
- How is the charity's VMMC programming implemented, and how does that compare to the implementation methods in the RCTs?
- Where would the charity allocate increased funding for VMMC, and, if it is allocating funding directly to surgeries, why is it confident there will be sufficient demand?
- What is the proportion of total unit cost of a circumcision that would be incurred if someone tested positive for HIV and was therefore not circumcised?
- What is the charity's rate of moderate and serious adverse events from VMMC surgery and how robust is its adverse event data?
- How does the charity communicate that VMMC is only partially protective to the populations it serves? Does the charity collect data about behavioral risk compensation?
In the future, we may also consider reviewing macro-evidence on the success of VMMC. This could involve searching for meta-analyses of observational studies on VMMC and looking at estimates of whether large-scale VMMC programs were followed by changes in HIV or cervical cancer incidence rates.
Sources
- 1
Tobian et al 2009 enrolled a subset (n=3393) of randomized, herpes-simplex virus type 2 negative participants from a VMMC trial in Uganda. The results were as follows: "At 24 months, the cumulative probability of HSV-2 [acquisition] was 7.8% in the intervention group and 10.3% in the control group (adjusted hazard ratio in the intervention group, 0.72; 95% confidence interval [CI], 0.56 to 0.92; P=0.008)." Tobian et al 2009, Abstract, Pg. 1298.
- 2
Sobngwi-Tambekou et al 2008 used urine samples collected from 1767 men during a VMMC trial in South Africa (Auvert et al 2005) to test the association between VMMC and neisseria gonorrhoeae, chlamydia trachomatis and trichomonas vaginalis. The study only found an association with trichomonas vaginalis, and this association was statistically significant in the as-treated analysis alone (Odds Ratio 0.49, p = 0.030; Adjusted Odds Ratio 0.41, p = 0.030). See Sobngwi-Tambekou et al 2008, Abstract, Pg. 116.
- 3
On VMMC's association with high-risk HPV, see:
- Auvert et al 2009 studied the prevalence of high-risk HPV in 1264 participants in the South Africa trial of VMMC (Auvert et al 2005). The authors found the prevalence of high-risk HPV was 14.8% in the intervention group and 22.3% in the control group, a prevalence rate ratio of 0.66 (95%CI: 0.51-0.86) over a 21-month period. See Auvert et al 2009, Abstract, Pg. 14 and "Methods: Collection of data", Pg. 15.
- Tobian et al 2009 studied the prevalence of high-risk HPV in 520 HIV-negative participants in the Uganda trials of VMMC (Gray et al 2007, Wawer et al 2009). The authors found the prevalence of high-risk HPV was 18% in the intervention group and 27.9% in the control group, an adjusted risk ratio of 0.65 (95%CI: 0.46-0.90). See Tobian et al 2009, Abstract, Pg. 1298.
On high-risk HPV's association with cancers in men: "Most men who get HPV (of any type) never develop any symptoms or health problems. But some types of HPV can cause genital warts. Other types can cause cancers of the penis, anus, or oropharynx (back of the throat, including base of the tongue and tonsils)", Centers for Disease Control and Prevention, HPV and Men Factsheet - 2012, Pg. 1.
- 4
- On HIV: "Globally, 35.0 million [33.2–37.2 million] people were living with HIV at the end of 2013", World Health Organization – Global Health Observatory Data – HIV/AIDS – 2016. GBD Compare - 2013 estimates that HIV caused approximately 70 million disability-adjusted life years (DALYs) in 2013. See graph here. We have not vetted this estimate.
- On cervical cancer: "Cervical cancer is the second most common cancer in women living in less developed regions with an estimated 445 000 new cases in 2012 (84% of the new cases worldwide)", World Health Organization – HPV Factsheet – 2015. GBD Compare - 2013 estimates that cervical cancer caused approximately 7 million DALYs in 2013. See graph here. We have not vetted this estimate.
- GBD Compare - 2013 estimates that genital herpes caused approximately 300,000 DALYs in 2013. See graph here. We have not vetted this estimate and we are not confident that all negative effects of herpes simplex virus type 2 (HSV-2) would be captured in this DALY estimate.
- GBD Compare - 2013 estimates that trichomoniasis caused approximately 100,000 DALYs in 2013. See graph here. We have not vetted this estimate.
- Parkin 2006 estimates that about 88% of HPV-attributable cancers were cervical cancers in 2002. See Table III of Parkin 2006, Pg. 3034. We divided the number of cases of cervical cancer by the total number of HPV-attributable cancers in Table III (492,800/561,200) and find that 87.8% of HPV-attributable cancers were cervical cancers in 2002. This implies that 12.2% of HPV-attributable cancers are not cervical cancers, but rather are cancers of the vulva, vagina, penis, anus, mouth, or oropharynx (as listed in the table). The relative proportions of HPV-attributable cancers may have changed since the publication of Parkin 2006, but we did not investigate this.
- 5
For calculations, see the our cost-effectiveness analysis of VMMC, "VMMC extraction + calcs" sheet, Section 2, “Weighted average” row. Data is sourced from World Health Organization - Data on Prevalence of HIV Among Adults Aged 15 to 49 - 2014 and Population Reference Bureau - World Population Sheet - 2013.
- 6
For calculations, see our cost-effectiveness analysis of VMMC, "VMMC extraction + calcs" sheet, Section 3, “Weighted average” row. Data is sourced from Institute for Health Metrics and Evaluation –HIV/AIDS Data for Download – 2013 and Population Reference Bureau - World Population Sheet - 2013.
- 7
- For calculations, see our cost-effectiveness analysis of VMMC, "VMMC extraction + calcs" sheet, Section 4, “Weighted average” row. Estimates are calculated using a weighted average of the cumulative incidence and mortality rates reported for Eastern, Middle and Southern Africa. Data is sourced from Arbyn et al 2011, Table 1, Pg. 2676.
- See also: GBD Compare - 2013, which estimated that in Sub-Saharan Africa cervical cancer is the cause of death for 1.2% of women aged 15-49 (see graph here), 4.13% of women aged 50-69 (see graph here), and 1.23% of women aged 70+ (see graph here). We have not vetted these estimates.
- 8
"HIV is a virus spread through certain body fluids that attacks the body's immune system, specifically the CD4 cells, often called T cells", Centers for Disease Control and Prevention – About HIV/AIDS – 2015.
- 9
"No effective cure currently exists for HIV. But with proper medical care, HIV can be controlled. Treatment for HIV is called antiretroviral therapy or ART", Centers for Disease Control and Prevention – About HIV/AIDS – 2015.
- 10
"HIV virus passes from one person to another through certain body fluids, such as blood and semen. About 90% of new HIV infections in the U.S. occur during sex", National Institutes of Health – HIV and AIDS: Know the Facts – 2015.
- 11
"When cancer starts in the cervix, it is called cervical cancer. The cervix is the lower, narrow end of the uterus", Centers for Disease Control and Prevention – Basic Information About Cervical Cancer - 2014.
- 12
"Almost all cervical cancers are caused by human papillomavirus (HPV), a common virus that can be passed from one person to another during sex", Centers for Disease Control and Prevention – What Are the Risk Factors for Cervical Cancer? - 2014.
- 13
"HPV types are often referred to as "low-risk" (wart-causing) or "high-risk" (cancer-causing), based on whether they put a person at risk for cancer. The International Agency for Research on Cancer found that 13 HPV types can cause cervical cancer", Centers for Disease Control and Prevention – Basic Information about HPV and Cancer – 2013.
- 14
"Studies have shown that more than 90% of new HPV infections, including those with high-risk types, clear or become undetectable within two years, and clearance usually occurs in the first 6 months after infection", Centers for Disease Control and Prevention - Vaccine Preventable Diseases Surveillance Manual - 2011, Ch. 5-1.
- 15
"Most people who become infected with HPV do not know they have it. … When the body's immune system can't get rid of a high-risk HPV infection, it can linger over time and turn normal cells into abnormal cells and then cancer. About 10% of women with high-risk HPV on their cervix will develop long-lasting HPV infections that put them at risk for cervical cancer", Centers for Disease Control and Prevention – Basic Information about HPV and Cancer – 2013.
- 16
"It takes 15 to 20 years for cervical cancer to develop in women with normal immune systems. It can take only 5 to 10 years in women with weakened immune systems, such as those with untreated HIV infection [sic]", World Health Organization – HPV Factsheet – 2015.
- 17
"Male circumcision is surgical removal of the foreskin - the retractable fold of tissue that covers the head of the penis", World Health Organization - Voluntary Medical Male Circumcision for HIV Prevention Factsheet – 2012.
- 18
"Male circumcision under local anaesthesia … [t]hree surgical techniques are described: the forceps-guided method; the dorsal slit method; [and] the sleeve resection method", World Health Organization – Manual for Male Circumcision under Local Anaesthesia – 2009, Chapter 5-1.
- 19
- "ShangRing" and "PrePex", World Health Organization – List of Pre-qualified Male Circumcision Devices - 2015.
- "WHO's list of prequalified medicinal products is used by international procurement agencies and increasingly by countries to guide bulk purchasing of medicines...WHO prequalification of medicines is a service provided by WHO to assess the quality, safety and efficacy of medicinal products. Originally, in 2001, the focus was on medicines for treating HIV/AIDS, tuberculosis and malaria. In 2006, this was extended to cover medicines and products for reproductive health and again in 2008, to cover prequalification of zinc, for managing acute diarrhoea in children. At the end of 2012, the WHO List of Prequalified Medicinal Products contained 316 medicines for priority diseases.
Every year, billions of US dollars worth of medicines are purchased by international procurement agencies for distribution in resource-limited countries. Prequalification is intended to give these agencies the choice of a wide range of quality medicines for bulk purchase." World Health Organization - Prequalification of medicines by WHO - 2013.
- 20
"VMMC has been incorporated into the HIV prevention portfolio and more than 9 million VMMCs have been performed. Conventional surgical procedures consist of forceps-guided, dorsal slit or sleeve resection techniques. Devices are also becoming available that might help to accelerate the scale-up of adult VMMC. The ideal device should make VMMC easier, safer, faster, sutureless, inexpensive, less painful, require less infrastructure, be more acceptable to patients and should not require follow-up visits. Elastic collar compression devices cause vascular obstruction and necrosis of foreskin tissue and do not require sutures or injectable anaesthesia. Collar clamp devices compress the proximal part of the foreskin to reach haemostasis; the distal foreskin is removed, but the device remains and therefore no sutures are required. Newer techniques and designs, such as tissue adhesives and a circular cutter with stapled anastomosis, are improvements, but none of these methods have achieved all desirable characteristics. Further research, design and development are needed to address this gap to enable the expansion of the already successful VMMC programmes for HIV prevention.", Tobian et al 2015, Abstract.
- 21
"In 2007 … WHO and UNAIDS recommended the intervention be added in countries with high HIV prevalence, generalized heterosexual HIV epidemics, and low levels of male circumcision where the intervention is likely to have the greatest public health impact. Fourteen priority countries with this profile are striving to scale up voluntary medical male circumcision: Botswana, Ethiopia, Kenya, Lesotho, Malawi, Mozambique, Namibia, Rwanda, South Africa, Swaziland, Tanzania, Uganda, Zambia and Zimbabwe", World Health Organization - Voluntary Medical Male Circumcision for HIV Prevention Factsheet – 2012.
- 22
- "In 2007 … WHO and UNAIDS recommended the intervention be added in countries with high HIV prevalence, generalized heterosexual HIV epidemics, and low levels of male circumcision where the intervention is likely to have the greatest public health impact. Fourteen priority countries with this profile are striving to scale up voluntary medical male circumcision: Botswana, Ethiopia, Kenya, Lesotho, Malawi, Mozambique, Namibia, Rwanda, South Africa, Swaziland, Tanzania, Uganda, Zambia and Zimbabwe", World Health Organization - Voluntary Medical Male Circumcision for HIV Prevention Factsheet – 2012.
- "In line with global goals such as Millennium Development Goal 6 to halt and reverse the spread of HIV and the WHO Global Health Sector Strategy on HIV/AIDS, a five-year Joint strategic action framework to accelerate the scale-up of voluntary medical male circumcision for HIV prevention in Eastern and Southern Africa 2012-2016 was developed by WHO and UNAIDS with the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR), the Bill & Melinda Gates Foundation and the World Bank and in consultation with national ministries of health. The framework calls for an intensified response by countries and partners to ‘catch up’ with men 15 to 49 years old who were not previously circumcised and to establish sustainable services for infants and adolescents for the longer term", World Health Organization - Voluntary Medical Male Circumcision for HIV Prevention Factsheet – 2012.
- 23
"The present comprehensive review highlights the reasons why the foreskin, in particular the inner mucosal lining, is the weak point that allows HIV to infect men during unprotected vaginal or anal intercourse with an infected partner", Morris and Wamai 2012, Pg. 158.
- 24
"We combined the survival estimates for all three trials at 12 months and also at 21 or 24 months in a meta-analysis using available case analyses using the random effects model. The resultant incidence risk ratio (IRR) was 0.50 at 12 months with a 95% confidence interval (CI) of 0.34 to 0.72; and 0.46 at 21 or 24 months (95% CI: 0.34 to 0.62)", Siegfried et al 2009, Abstract, Pg. 2.
- 25
"Three large RCTs of men from the general population were conducted in South Africa (N = 3 274), Uganda (N = 4 996) and Kenya (N = 2 784) between 2002 and 2006", Siegfried et al 2009, Pg. 2.
- 26
"A total of 3,274 uncircumcised men, aged 18–24 y, were randomized to a control or an intervention group with follow-up visits at months 3, 12, and 21", Auvert et al 2005, Pg. 1112.
- 27"A randomized, controlled, blindly evaluated intervention trial was carried out in Orange Farm and surrounding areas, a semi-urban region close to the city of Johannesburg", Auvert et al 2005, Pg. 1113.
- 28"There were 20 HIV infections (incidence rate = 0.85 per 100 person-years) in the intervention group and 49 (2.1 per 100 person-years) in the control group", Auvert et al 2005, Pg. 1112.
- 29
"There were 20 HIV infections (incidence rate = 0.85 per 100 person-years) in the intervention group and 49 (2.1 per 100 person-years) in the control group", Auvert et al 2005, Pg. 1112.
- 30
"The trial was stopped at the interim analysis, and the mean (interquartile range) follow-up was 18.1 mo (13.0–21.0) when the data were analyzed", Auvert et al 2005, Pg. 1112.
- 31
"This RR corresponds to a protection of 60% (95% CI: 32%–76%)", Auvert et al 2005, Pg. 1112.
- 32"We did a randomised controlled trial of 2784 men aged 18–24 years in Kisumu, Kenya", Bailey et al 2007, Pg. 643.
- 33"We did a randomised controlled trial of 2784 men aged 18–24 years in Kisumu, Kenya", Bailey et al 2007, Pg. 643.
- 34"The 2-year HIV incidence was 2.1% (95% CI 1.2–3.0) in the circumcision group and 4.2% (3.0–5.4) in the control group (p=0.0065)", Bailey et al 2007, Pg. 643.
- 35"The 2-year HIV incidence was 2.1% (95% CI 1.2–3.0) in the circumcision group and 4.2% (3.0–5.4) in the control group (p=0.0065)", Bailey et al 2007, Pg. 643.
- 36"The median length of follow-up was 24 months", Bailey et al 2007, Pg. 643.
- 37"[T]he relative risk of HIV infection in circumcised men was 0.47 (0.28–0.78), which corresponds to a reduction in the risk of acquiring an HIV infection of 53% (22–72)", Bailey et al 2007, Pg. 643.
- 38"4996 uncircumcised, HIV-negative men aged 15–49 years who agreed to HIV testing and counselling were enrolled in this randomised trial in rural Rakai district, Uganda", Gray et al 2007, Pg. 657.
- 39"4996 uncircumcised, HIV-negative men aged 15–49 years who agreed to HIV testing and counselling were enrolled in this randomised trial in rural Rakai district, Uganda", Gray et al 2007, Pg. 657.
- 40"In the modified intention-to-treat analysis, HIV incidence over 24 months was 0.66 cases per 100 person-years in the intervention group and 1.33 cases per 100 person-years in the control group (estimated efficacy of intervention 51%, 95% CI 16–72; p=0.006)", Gray et al 2007, Pg. 657.
- 41"In the modified intention-to-treat analysis, HIV incidence over 24 months was 0.66 cases per 100 person-years in the intervention group and 1.33 cases per 100 person-years in the control group (estimated efficacy of intervention 51%, 95% CI 16–72; p=0.006)", Gray et al 2007, Pg. 657.
- 42"In the modified intention-to-treat analysis, HIV incidence over 24 months was 0.66 cases per 100 person-years in the intervention group and 1.33 cases per 100 person-years in the control group (estimated efficacy of intervention 51%, 95% CI 16–72; p=0.006)", Gray et al 2007, Pg. 657.
- 43"In the modified intention-to-treat analysis, HIV incidence over 24 months was 0.66 cases per 100 person-years in the intervention group and 1.33 cases per 100 person-years in the control group (estimated efficacy of intervention 51%, 95% CI 16–72; p=0.006)", Gray et al 2007, Pg. 657.
- 44
Table 1, Auvert et al 2005, Pg. 1113; "Panel: inclusion and exclusion criteria", Bailey et al 2007, Pg. 644.
- 45
"4996 uncircumcised, HIV-negative men aged 15–49 years who agreed to HIV testing and counselling were enrolled in this randomised trial in rural Rakai district, Uganda", Gray et al 2007, Pg. 657.
- 46
"Quality of the Data, Blinding, Confidentiality, and Data Management", Auvert et al 2005, Pg. 1114.
- 47
"The nurse-counsellors who did the HIV testing and administered the questionnaire were blinded to study group, unless the participant divulged his circumcision status during counselling", Bailey et al 2007, Pg. 645.
- 48
"Procedures", Gray et al 2007, Pg 658.
- 49
- "Quality of the Data, Blinding, Confidentiality, and Data Management", Auvert et al 2005, Pg. 1114; "Procedures", Gray et al 2007, Pg. 658.
- Siegfried et al 2009 graded allocation concealment in Auvert et al 2005 and Gray et al 2007 as having "High risk of bias." See Figures 1 and 2, Siegfried et al 2009, Pgs. 7-8.
- "Allocation concealment
- Adequate: participants and the investigators enrolling participants cannot foresee assignment (e.g., central allocation; or sequentially numbered, opaque, sealed envelopes).
- Inadequate: participants and investigators enrolling participants can foresee upcoming assignment (e.g., an open random allocation schedule, a list of random numbers); or envelopes were unsealed or nonopaque or not sequentially numbered.
- Unclear: insufficient information to permit judgement of the allocation concealment or the method not described." Siegfried et al 2009, Pg. 5.
- Details on allocation concealment methods, according to Siegfried et al 2009:
- Auvert et al 2005: "Envelopes described as “sealed.” Participants were asked to select one envelope from a basket of 10, after which the envelope was replaced by the next sequential envelope from a set of pre-prepared envelopes that contained five for the control and five for the intervention arm, suggesting use of blocking, although this is not clearly stated. The final numbers between the two groups differ by 34, suggesting that imbalances occurred with this method of randomisation and allocation. As the process allowed participants to pick envelopes, this would not ensure that the envelopes were opened one at a time and used in order. Therefore, the procedure must be graded as inadequate" Siegfried et al 2009, Pgs. 15-16.
- Gray et al 2007: Envelopes described as “opaque.” Blocks of 20 envelopes were stratified on community, with no report of how many communities were involved. Participants were asked to select one envelope from a box of 20, after which the envelope was replaced by the next envelope from the next batch for that community. The trialists recognise that this procedure resulted in temporary imbalances between study groups, thereby reducing the blocking effect. As the process allowed participants to pick envelopes, this would not ensure that the envelopes were opened one at a time and used in order. Therefore the procedure must be graded as inadequate" Siegfried et al 2009, Pg. 19.
- 50
"Procedures", Bailey et al 2007, Pg. 645; See also "As Bailey 2007 did not explicitly report using sealed envelopes, although the process was otherwise adequately reported, we have marked allocation concealment as unclear", Siegfried et al 2009, Pg. 8.
- 51
"The circumcisions were performed by three local general practitioners in their surgical offices. The general practitioners were experienced in MC practices. … The procedure was standardized and used the forceps-guided method", Auvert et al 2005, Pg. 1114.
- 52
"Circumcisions were done by trained and certified physicians in well-equipped operating theatres with careful attention to asepsis… Circumcision was done with the sleeve procedure, in which the foreskin was retracted and a distal incision made 0.5–1.0 cm proximal to the coronal sulcus, followed by a proximal incision on the unretracted prepuce at the corona. … Postoperative follow-up visits were scheduled at 24–48 hours, 5–9 days, and 4–6 weeks", Gray et al 2007, Pg. 658.
- 53
"All surgeries were done under local anaesthesia in the study clinic by study clinicians, using the standardised forceps-guided method described by Krieger and colleagues … Post circumcision visits were scheduled for 3, 8, and 30 days", Bailey et al 2007, Pg. 645.
- 54
- "At each of the four visits, each participant was invited to answer a face-to-face questionnaire, to provide a blood sample, and to have a genital examination and an individual counselling session … The counselling session (15–20 min) was delivered by a certified counsellor and focused on information about STIs in general and HIV in particular and on how to prevent the risk of infection. During this session, participants were encouraged to attend voluntary counselling and testing in a public clinic located 200 m away from the investigation centre or in a voluntary counselling and testing (VCT) centre funded by the project and located in the same building as the investigation centre. Condoms were provided in the waiting room of the investigation centre and were also provided by the counsellor", Auvert et al 2005, Pg. 1114.
- "At each follow up … repeat HIV counselling and testing and health education were provided", Gray et al 2007, Pg. 658- 659.
- "Individually tailored risk reduction counselling occurred at every visit. Men who tested positive for a sexually transmitted infection were treated, received additional counselling, and were given a coupon for their sexual partner to receive free treatment at a neighbouring public clinic. Incident HIV-positive men were referred to the project’s post-test counselling and support group and provided access to free HIV treatment and care", Bailey et al 2007, Pg. 645.
- 55
According to Siegfried et al 2009:
- Auvert et al 2005 employed the following testing procedure: "HIV-1 established by an ELISA screen plus two confirmatory tests. All three tests were required to be positive in order to be classified as HIV-positive", Pg. 15.
- Bailey et al 2007 employed the following testing procedure: "HIV-1 and HIV-2 established by using the Determine HIV 1/2 rapid testing on finger-prick blood. If positive on two tests or if discordant, serum was sent for a double ELISA. Participants were deemed to be HIV-positive if both ELISA tests were positive. Discordant tests were indeterminate and participants were asked to return for additional testing from one to six months later, depending on the visit", Pg. 17.
- Gray et al 2007 employed the following testing procedure: "HIV-1 established by two ELISA tests and for discordant results, a confirmatory Western blot. For those who seroconverted during follow-up, the previous negative sample and in selected cases (not detailed) the first positive sample, were tested by polymerase chain reaction (PCR)", Pg. 19.
- 56
"The participants received a total of 300 South African Rand [~$53.29USD] as compensation", Auvert et al 2005, Pg. 1113. (According to XE.com's current and historical rate tables, on 1 January 2005 one ZAR was worth 0.1776198973 USD.)
- 57
"Men received US$5 at screening and enrolment, $5 at the time of surgery, and $5 on completion of postoperative follow-up. Control participants who were circumcised at completion of their 24 months of follow-up received identical compensation. The amount of compensation for routine follow-up visits at 6, 12, and 24 months was $3 per visit", Gray et al 2007, Pg. 659.
- 58
"Participants were offered 300 Kenyan shillings (about $4) for each scheduled study visit to cover travel expenses and loss of income", Bailey et al 2007, Pg. 644.
- 59
- "The grouped censored data were analyzed in intention-to-treat, univariate and multivariate, analyses, using piecewise exponential, proportional hazards models", Auvert et al 2005, Pg. 1112.
- "The primary modified intention-to-treat population included crossovers and participants who reported periods of sexual abstinence during the 24 months of follow-up", Gray et al 2007, Pg. 660.
- "The primary analysis was by intention-to-treat; participants were included in the analysis in the group to which they were randomly assigned and all participants with follow-up for HIV status were included in the analysis", Bailey et al 2007, Pg. 646.
- 60
"In the intervention group, 6.5% (93/1432) were not circumcised at M3, and in the control group, 10.3% (114/1105) were circumcised at M21 (Figure 1).", Auvert et al 2005, Pg. 1117.
- 61
See Figure 1, Gray et al 2007, Pg. 659.
- 62
See Figure 1, Bailey et al 2007, Pg. 647.
- 63
- "The South African trial was stopped early in April 2005 by the Institutional
Monitoring and Safety Board after interim results exceeded the limits of the early stopping rule. The Kenyan trial and the Ugandan trial were also stopped early in December 2006 for the same reason", Siegfried et al 2009, Pg. 6. - "Each trial employed early stopping rules considered acceptable statistical practice", Siegfried et al 2009, Pg. 11. We have not vetted this statement.
- "Male Circumcision for HIV Prevention in Rakai, Uganda … Study Start Date: August 2002; Estimated Study Completion Date: December 2006", ClinicalTrials.gov - NCT00425984.
- "Male Circumcision and HIV Rates in Kenya … Study Start Date: February 2002 … Study Completion Date: December 2006", ClinicalTrials.gov - NCT00059371.
- "Effect of Male Circumcision on HIV Incidence (ANRS 1265) … Study Start Date: July 2002 … Study Completion Date: July 2005", ClinicalTrials.gov - NCT00122525.
- "The South African trial was stopped early in April 2005 by the Institutional
- 64
"Stopping each of the three trials early, however, resulted in high attrition within each trial, ranging from 30% to 46%. Use of survival analysis, which incorporates the results from all of those who completed the trial and who are censored due to loss to follow-up or early stopping of the trial, will have reduced the potential attrition bias in each trial. This was done in each trial", Siegfried et al 2009, Pg. 11.
- 65
- "When trials with events fewer than the median number (n=66) were compared with those with event numbers above the median, the odds ratio for a magnitude of effect greater than the median [magnitude of effect] was 28 (95% CI 11 to 73)", Siegfried et al 2009, Pg. 11 referring to "Systematic review up to November 2004 … [of] 143 RCTs stopped early for benefit", Montori et al 2005, Pg. 2203.
- There were a relatively low number of events (i.e., HIV infections) in the three core VMMC RCTs. See, e.g., in one of the RCTs: "There were 20 HIV infections (incidence rate = 0.85 per 100 person-years) in the intervention group and 49 (2.1 per 100 person-years) in the control group", Auvert et al 2005, Pg. 1112.
- 66
"However, the magnitude of effect was consistent across all three trials, which strengthens the evidence in favour of male circumcision", Siegfried et al 2009, Pg. 11.
- 67
"We rated the risk of bias due to incomplete outcome reporting as moderate in all three trials, as acceptable statistical survival analysis techniques were used to estimate HIV event distribution over time by accumulating for staggered enrolment and incomplete discrete follow-up.", Siegfried et al 2009, Pg. 9.
- 68
- "ANRS 1265 [ie, Auvert et al 2005] (Continued) ... Loss to follow-up, including those who were HIV-positive, was 30.4% for the circumcised group and 33.4% for the uncircumcised group. Survival analysis was used to incorporate censored outcome data in the analysis", Siegfried et al 2009, Pg. 16.
- "Overall, follow-up for HIV status was incomplete for 240 (8·6%) participants: 126 (9·1%) in the circumcision group and 114 (8·2%) in the control group. There were no significant differences in the event distribution with time for the missed visits.", Bailey et al 2007, Pg. 648.
- "By December 12, 2006, the date of trial termination, 44% of men in both groups had reached their 24 month follow-up time point; retention rates for these men were much the same in both groups.", Gray et al 2007, Pg. 663.
- 69
Siegfried et al 2009, Pg. 2.
- 70
- Results: "In weight-adjusted Cox regression, the hazard ratio was 0.42 [95% CI: 0.26–0.66]. … Conclusion: The efficacy of MMC was sustained at 58% at 72 months", Mehta et al 2013, Pg. 2899.
- Study sample: "Of the 1740 men still enrolled [after the trial was stopped] and eligible to participate in extended follow-up, 1545 (89%) provided additional consent to do so. Of the 1545 men consenting to extended follow-up, 778 (50.4%) were initially randomized to the control group. The follow-up visit schedule and procedures were identical to those of the trial, with scheduled visits every 6 months that included personal interview, physical examination, and STI and HIV testing. Extended follow-up was completed on 30 September 2010, and the remaining cohort was discharged at that time", Mehta et al 2013, Pg. 2900-1.
- Attrition: "Posttrial retention among men consenting to extended follow-up was 84% at 36 months, 72% at 48 months, 68% at 60 months, and 52% at 72 months." Attrition was significantly higher in the control arm, from which participants were removed as they became circumcised: "Within 12 months of the end of the trial, 24% of the men randomized to delayed circumcision became circumcised, and by the end of the extended follow-up period, approximately 50% (n=395) had done so", Mehta et al 2013, Pg. 2901. By 72 months, the control group had 85 participants (of 1383) and the intervention group had 290 (of 1306), meaning there was 1 control group participant for every 3.41 intervention group participants.
- Methodology: To estimate the effect size, the authors use a marginal structural model to apply weights at each time point to reduce the bias from time-dependent confounders, such as men choosing to be circumcised or unexplained loss to follow-up. To obtain stabilized weights, the authors use a pooled logistic regression model for circumcision using each 6-month study visit as an observation. Finally, the authors also used a Cox proportional hazards model to incorporate the risk of other covariates with HIV infection over time (See "Marginal structural model analysis", Mehta et al 2013, Pg. 2901).
- 71
"Following trial closure we offered MC to uncircumcised control arm participants maintained surveillance for up to 4.79 years", Gray et al 2012.
- 72
- On methods: "Following trial closure we offered MC to uncircumcised control arm participants … HIV incidence per 100 person-years (py) was assessed in an as-treated analysis, and the effectiveness of MC was estimated using Cox regression models, adjusted for sociodemographic and time-dependent sexual behaviors", Gray et al 2012. Cox regression models adjust the hazard ratio to account for covariates which change over time. We found the methods section opaque as to what statistical adjustments were made to account for participants being circumcised at different points in time, and for the significant numerical imbalance between treatment and control groups. In our view, the clearest statement about these potential biases is an acknowledgement by the authors that the higher effectiveness recorded after the trial ended (which was not statistically significant) may reflect some unmeasured self-selection by men choosing not to accept VMMC. See: "It is possible that the higher effectiveness after than during the trial, although not statistically significant, reflects some unmeasured self-selection of men choosing not to accept MC. … At trial closure, there were no statistically significant differences in sociodemographic characteristics or sexual risk behaviors among men who opted for MC compared to those who declined MC, except that men who opted for MC were slightly more likely to report no sexual activity in the prior 12 months (14.3%) than those declining MC (10.6% p=0.08)", Gray et al 2012.
- On results: "In control arm participants, post-trial HIV incidence was 0.54/100 py in circumcised and 1.71/100 py in uncircumcised control arm men (adjusted effectiveness 67% (95%CI 38-83%))", Gray et al 2012.
- On the study population: "78.4% of uncircumcised trial participants accepted MC following trial closure", Gray et al 2012.
- On attrition: "Retention during post-trial surveillance was 74%", Gray et al 2012.
- On potential under-estimation bias: "There are also inherent limitations to the interpretation of the post-trial data. Men who were circumcised during the post-trial period spent part of each follow up interval in an uncircumcised state. Although we know the dates of circumcision, we do not know when HIV infection occurred within a follow up interval, so incident infections cannot be definitively ascribed to an uncircumcised or circumcised state. Thus, if men were circumcised during a follow up interval when they seroconverted, we conservatively assumed the HIV infection occurred after circumcision although the HIV infection may have preceded the procedure. This may have misclassified circumcision status for some incident HIV infections and biased our estimates towards the null", Gray et al 2012.
- 73
“The Bophelo Pele project is a community-based campaign against HIV, which includes the roll-out of free VMMC. A baseline cross-sectional biomedical survey was conducted in 2007–2008 among a random sample of 1,998 men aged 15 to 49 (survey response rate 80.7%). In 2010–2011, we conducted a follow-up random survey among 3,338 men aged 15 to 49 (survey response rate 79.6%) to evaluate the project. Participants were interviewed, blood samples were collected and tested for HIV and recent HIV infection (using the BED HIV incidence assay), and MC status was assessed through a clinical examination. Data were analyzed using multivariate and propensity statistical methods.
Owing to the VMMCs performed in the context of the RCT and the Bophelo Pele project, the prevalence rate of adult MC increased from 0.12 (95% CI 0.10–0.14) to 0.53 (95% CI 0.51–0.55). Without these VMMCs, the HIV prevalence rate in 2010–2011 would have been 19% (95% CI 12%–26%) higher (0.147 instead of 0.123).
When comparing circumcised and uncircumcised men, no association of MC status with sexual behavior was detected. Among circumcised and uncircumcised men, the proportion consistently using condoms with non-spousal partners in the past 12 months was 44.0% (95% CI 41.7%–46.5%) versus 45.4% (95% CI 42.2%–48.6%) with weighted prevalence rate ratio (wPRR) = 0.94 (95% CI 0.85–1.03). The proportion having two or more non-spousal partners was 50.4% (95% CI 47.9%–52.9%) versus 44.2% (95% CI 41.3%–46.9%) with wPRR = 1.03 (95% CI 0.95–1.10).
We found a reduction of BED-estimated HIV incidence rate ranging from 57% (95% CI 29%–76%) to 61% (95% CI 14%–83%) among circumcised men in comparison with uncircumcised men…Findings suggest that the roll-out of VMMC in Orange Farm is associated with a significant reduction of HIV levels in the community. The main limitation of the study is that it was not randomized and cannot prove a causal association. The roll-out of VMMC among adults in sub-Saharan Africa should be an international priority and needs to be accelerated to effectively combat the spread of HIV.” Abstract, Auvert et al 2013.
- 74
"We found no completed RCT and included 21 observational studies with 71,693 participants. The only eligible RCT is currently ongoing among MSM in China. The pooled effect estimate for HIV acquisition was not statistically significant (20 studies; 65,784 participants; OR 0.86, 95% CI 0.70 to 1.06) and showed significant heterogeneity (I^2=53%)", Wiysonge et al 2011, Abstract.
- 75
"In a subgroup analysis, the results were statistically significant in studies of men reporting an insertive role (7 studies, 3465 participants; OR 0.27, 95% CI 0.17 to 0.44; I2= 0%)", Wiysonge et al 2011, Abstract.
- 76
"922 uncircumcised, HIV-infected, asymptomatic men aged 15–49 years with CD4-cell counts 350 cells per μL or more were enrolled in this unblinded, randomised controlled trial in Rakai District, Uganda. Men were randomly assigned by computer-generated randomisation sequence to receive immediate circumcision (intervention; n=474) or circumcision delayed for 24 months (control; n=448). HIV-uninfected female partners of the randomised men were concurrently enrolled (intervention, n=93; control, n=70) and followed up at 6, 12, and 24 months, to assess HIV acquisition by male treatment assignment (primary outcome)", Wawer et al 2009, Pg. 229.
- 77
"The trial was stopped early because of futility. 92 couples in the intervention group and 67 couples in the control group were included in the modified ITT analysis. 17 (18%) women in the intervention group and eight (12%) women in the control group acquired HIV during follow-up (p=0.36). … Circumcision of HIV-infected men did not reduce HIV transmission to female partners over 24 months; longer-term effects could not be assessed", Wawer et al 2009, Pg. 229.
- 78
"Almost all cervical cancers are caused by human papillomavirus (HPV), a common virus that can be passed from one person to another during sex", Centers for Disease Control and Prevention – What Are the Risk Factors for Cervical Cancer? - 2014.
- 79
See, eg, "The biological mechanism through which male circumcision could reduce HPV infection rates in female partners probably involves a reduction of penile HPV carriage", Wawer et al 2011, Pg. 217.
- 80
- Auvert et al 2009 studied the prevalence of high-risk HPV in 1264 participants in the South Africa trial of VMMC (Auvert et al 2005). The authors found the prevalence of high-risk HPV was 14.8% in the intervention group and 22.3% in the control group, a prevalence rate ratio of 0.66 (95%CI: 0.51-0.86) over a 21-month period. See Auvert et al 2009, Abstract, Pg. 14 and "Methods: Collection of data", Pg. 15.
- Tobian et al 2009 studied the prevalence of high-risk HPV in 520 HIV-negative participants in the Uganda trials of VMMC (Gray et al 2007, Wawer et al 2009). The authors found the prevalence of high-risk HPV was 18% in the intervention group and 27.9% in the control group, an adjusted risk ratio of 0.65 (95%CI: 0.46-0.90). See Tobian et al 2009, Abstract, Pg. 1298 and Table 3, Pg. 1308.
- 81
Note the high-risk HPV genotypes assessed were "genotypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68", Wawer et al 2011, Pg. 212.
- 82
"During the trial, incidence of high-risk HPV infection in women was lower in the intervention group than in the control group (20.7 infections vs 26.9 infections per 100 person-years; incidence rate ratio=0.77, 0.63–0.93, p=0.008)", Wawer et al 2011, Pg. 209.
- 83
"After we adjusted for baseline age, education, number of sexual partners, alcohol consumption with sex, and condom use, and accounted for possible correlation of a woman clearing more than one genotype, cumulative high-risk HPV clearance was higher in women in the intervention group than it was in women in the control group (RR 1.10, 95% CI 1.03–1.20, p=0.003). Clearance at year 2 of high-risk HPV infections (with all genotypes) acquired during the first year of the trial was also more likely in women in the intervention group (82%, n=124) than in women in the control group (70%, n=127; RR=1.17, 95% CI 1.04–1.32, p=0.014)", Wawer et al 2011, Pg. 215.
- 84
"Incident HPV was defined as a newly detected genotype identified in women whose swab was negative for any HPV at the previous study visit, or women who had previously had a positive result for HPV but had at least one newly detected HPV genotype during the next follow-up period. … each woman with a newly detected HPV genotype was counted only once per follow-up interval, irrespective of whether one or several HPV genotypes were detected", Wawer et al 2011, Pg. 213.
- 85
"Clearance (ie, loss of detection) of high-risk HPV was estimated in women with pre-existing high-risk HPV genotype-specific infections, and high-risk HPV genotype was the unit of observation. Clearance was expressed as the proportion of pre-existing high-risk HPV genotype-specific infections that were negative for that genotype at a subsequent study visit. Clearance was assessed for each high-risk HPV genotype, and all genotype-specific clearance events were combined to provide overall estimates", Wawer et al 2011, Pg. 213.
- 86
"Two parallel but independent trials of male circumcision for the prevention of HIV and other sexually transmitted infections were done in Rakai, Uganda … The first trial enrolled 4996 uncircumcised men who did not have HIV infection … The primary aim was to assess the effectiveness of male circumcision for the prevention of HIV infection … The second trial enrolled 922 men with HIV infection and 600 men without HIV infection … It aimed primarily to assess the efficacy of male circumcision for the prevention of HIV transmission to female partners", Wawer et al 2011, Pg. 210.
- 87
"…we assessed the effectiveness of male circumcision to prevent high-risk HPV infection in HIV-negative female partners of HIV-negative men who were enrolled in two randomised controlled trials of male circumcision in Rakai, Uganda. … Men who were married or in long-term consensual relationships were asked to identify their female partners, who were contacted separately and invited to participate in a follow-up study", Wawer et al 2011, Pg. 210.
- 88
"Men and women who had HIV infection at enrollment, or who acquired HIV infection during the trial, were excluded from analysis because HIV infection increases the risk of persistent HPV. During the course of the trial, more men in the control group than in the intervention group seroconverted to HIV; inclusion of HIV seroconverters in analyses could therefore have biased HPV results", Wawer et al 2011, Pg. 210.
- 89
"To ascertain a woman's baseline HPV status immediately before her partner was circumcised, and to avoid potential bias between groups, analysis was restricted to women who enrolled at the same time as their male partners", Wawer et al 2011, Pg. 210.
- 90
"HIV-uninfected female partners (648 of men from the intervention group, and 597 of men in the control group) were simultaneously enrolled", Wawer et al 2011, Pg. 209.
- 91
"All participants were offered free voluntary HIV counselling and testing", Wawer et al 2011, Pg. 210.
- 92
"To ascertain a woman’s baseline HPV status immediately before her partner was circumcised, and to avoid potential bias between groups, analysis was restricted to women who enrolled at the same time as their male partners." Wawer et al 2011, Pg. 210.
- 93
See Tables 1 and 2, Wawer et al 2011, Pg. 212-3.
- 94
"We performed a meta-analysis of published data to compare HPV type distribution in [high-grade squamous intraepithelial lesions (HSIL)] to [squamous cell carcinoma of the cervix (SCC)]. HPV 16, 18 and 45 were each more prevalent in SCC than HSIL … These data suggest that HSILs infected with HPV16, 18 and 45 preferentially progress to SCC," Clifford et al 2003, Pg. 101.
- 95
See Table 7, Wawer et al 2011, Pg. 217. Only 52% cleared HPV-16 in the intervention group, whereas 74% cleared it in the control group (risk reduction of 0.70 (95% CI: 0.54–0.92)).
- 96
"Men who were married or in long-term consensual relationships were asked to identify their female partners, who were contacted separately and invited to participate in a follow-up study. Women were eligible for enrolment if they provided informed consent and their male partner was a trial participant. ... Men and women who had HIV infection at enrolment, or who acquired HIV infection during the trial, were excluded from analysis because HIV infection increases the risk of persistent HPV." Wawer et al 2011, Pg. 210.
- 97
"People with weak immune systems may be less able to fight off HPV and more likely to develop health problems from it, this includes people with HIV/AIDS", Centers for Disease Control and Prevention – Genital HPV Infection Factsheet – 2014, Pg. 2.
- 98
"Men and women who had HIV infection at enrolment, or who acquired HIV infection during the trial, were excluded from analysis because HIV infection increases the risk of persistent HPV." Wawer et al 2011, Pg. 210.
- 99
"During the course of the trial, more men in the control group than in the intervention group seroconverted to HIV", Wawer et al 2011, Pg. 210.
- 100
At 24 months, data were available for 544/648 in the intervention group (83.9%) and 488/597 in the control group (81.7%). See "HIV-uninfected female partners (648 of men from the intervention group, and 597 of men in the control group) were simultaneously enrolled" and "At 24-month follow-up, data were available for 544 women in the intervention group and 488 in the control group", Wawer et al 2011, Pg. 209.
- 101
"21 adverse events among 20 participants (1.5%, 95% CI 0.9–2.3) were probably or definitely related to surgery. All were mild or moderate in severity. None was judged to be severe, and, except for the case of erectile dysfunction, all adverse events resolved with treatment within hours or days. … Adverse event rates were comparable with rates documented for neonatal circumcision in developed countries", Bailey et al 2007, Pg. 652-3.
- 102
"The 1.5% rate of adverse events in our study was lower than the 3.6% rate in Orange Farm. Both studies used much the same forceps-guided method. The difference in rates could be a result of multiple factors: all procedures in Kisumu were done at our study clinic by our own, highly trained and experienced practitioners; we had regular surgical case conferences to review outcomes; participants were given clear written postoperative instructions; and participants had scheduled clinic visits 3, 8, and 30 days after the procedure. The Orange Farm trial contracted experienced local private practitioners to do the operations in their own offices, and patients were seen only if they came back with a complication. The Orange Farm trial might more closely resemble what the situation is likely to be under non-study conditions", Bailey et al 2007, Pg. 653.
- 103
"The circumcisions were performed by three local general practitioners in their surgical offices. The general practitioners were experienced in MC practices", Auvert et al 2005, Pg. 1114.
- 104
"Adverse events (AEs) were documented and analyzed for all participants, including those who were HIV-positive at randomization. These AEs related to surgery, and that occurred in the first month post-surgery, were reported by the practitioners using a specific form", Auvert et al 2005, Pg. 1115.
- 105
Table 5, Auvert et al 2005, Pg. 1119.
- 106
"Circumcisions were done by trained and certified physicians in well-equipped operating theatres with careful attention to asepsis … Postoperative follow-up visits were scheduled at 24–48 hours, 5–9 days, and 4–6 weeks", Gray et al 2007, Pg. 658.
- 107
"Potential adverse events related to surgery were predefined and graded as mild (requiring no treatment), moderate (requiring treatment), or severe complications (requiring surgical intervention [eg, wound exploration for active bleeding, repair of wound dehiscence], hospitalisation, or referral for specialised care)", Gray et al 2007, Pg. 658.
- 108
"The rate of all adverse events related to surgery in the intervention group was about 8% (178 events in 2328 surgeries); most of these events were mild (94 of 178 events). The rate of moderate adverse events related to surgery was about 3% (79 events in 2328 surgeries), and there were five severe adverse events, with a rate of 0.2 events per 100 surgeries. The severe adverse events included one wound infection, two haematomas that required re-exploration and ligation of active bleeding vessels, one wound disruption due to external cause, and one case of severe postoperative herpetic ulceration not involving the surgical wound requiring hospitalisation in the programme's facility. All moderate and severe adverse events were successfully managed and resolved", Gray et al 2007, Pg. 664.
- 109
"… 88 subjects in our sample underwent the procedure medically … The AE rate among medical circumcisions was significantly lower (17.7%), but nevertheless very high compared to rates observed in developed countries and in clinical settings in Nigeria and Kenya", Bailey and Egesah 2006, Pg. 15.
- 110
We note Ford, Chu and Mills 2012, a systematic review of VMMC safety outcomes following task-shifting, namely the planned delegation of tasks from higher level health cadres (specialists or doctors) to non-physician clinicians. The abstract states: "Ten studies met our inclusion criteria, providing outcome data on 25119 circumcisions. The proportion of adverse events ranged from 0.70 [95% confidence interval (CI) 0.44–1.02%] to 37.36% (95% CI 27.54–47.72%), with an overall pooled proportion of 2.31% (95% CI 1.46 – 3.16%; t2 = 1.21; P < 0.001). Two studies reported outcomes separately for both doctors and non-physicians; there was no difference in the risk of adverse events by provider (pooled relative risk 1.18; 95% CI 0.78–1.78). … All adverse events were reported to be non-severe", Ford, Chu and Mills 2012, Pg. 559.
- 111
"We combined the survival estimates for all three trials at 12 months and also at 21 or 24 months in a meta-analysis using available case analyses using the random effects model. The resultant incidence risk ratio (IRR) was 0.50 at 12 months with a 95% confidence interval (CI) of 0.34 to 0.72; and 0.46 at 21 or 24 months (95% CI: 0.34 to 0.62)", Siegfried et al 2009, Abstract, Pg. 2.
- 112
"This is the first study to examine longitudinal change in HIV-associated risk behaviors in men before and after circumcision in the context of a large population-level VMMC program. We observed no evidence of behavioral risk compensation over 24 months of follow-up. Further, there is evidence that men exposed to the VMMC program, both as circumcised clients and through informational messages as study controls, meaningfully shifted towards safer behaviors. This behavioral reduction in risk was noted in all sexual risk behaviors examined, including increased condom use", Westercamp et al 2014.
- 113
See Table 4, Auvert et al 2005, Pg. 1118.
- 114
"Of the five reported sexual behavioural factors, all were higher in the intervention group than in the control group during the period M4–M12, and four out of five were higher during the period M13–M21", Auvert et al 2005, Pg. 1118.
- 115
"Only the mean number of sexual contacts showed statistically significant differences during the period M4–M12 (5.9 versus 5.0, p < 0.001) and during the period M13–M21 (7.5 versus 6.4, p = 0.0015)", Auvert et al 2005, Pg. 1118.
- 116
See Table 6, Gray et al 2007, Pg. 664; Table 1, Bailey et al 2007, Pg. 650 and Table 4, Bailey et al 2007, Pg. 652.
- 117
"There is, therefore, no consistent or substantial evidence of behavioural disinhibition after circumcision in the study population", Gray et al 2007, Pg. 664. Also see Table 6, Gray et al 2007, Pg. 664.
- 118
See Table 4, Bailey et al 2007, Pg. 652.
- 119
"Circumcised men exhibited slightly riskier behaviour on all five assessed measures at month 24 and this was significant for two of the measures—unprotected sexual intercourse with any partner in the previous 6 months and consistent condom use—at that time point. However, the differences between the two groups are attributable to increases in safer sexual practices in the control group rather than to riskier behaviour patterns in the circumcision group, indicating that risk compensation (ie, behavioural disinhibition) did not occur during the 24 months of this study", Bailey et al 2007, Pg. 654.
- 120
See, e.g., "This study confirms the importance of the foreskin for penile sensitivity, overall sexual satisfaction, and penile functioning", Bronselaer et al 2013, Pg. 820.
- 121
See, e.g., "Little is known about the long-term implications of neonatal circumcision on the penile sensitivity of adult men, despite recent public policy endorsing the procedure in the United States", Bossio, Pukall and Steele 2015, Abstract.
- 122
See our cost-effectiveness analysis of VMMC, “VMMC” sheet, “VMMC vs cash” row.
- 123
Our estimates of what portion of HIV-positive people do not receive antiretroviral therapy, and what portion of the population not receiving antiretroviral therapy dies over a particular timeframe are discussed below. We have not carefully vetted them. Additional research that we could do to refine these estimates and their relation to VMMC include:
- What portion of the deaths over the 10 year period in Van der Paal et al 2007 were due to causes other than HIV?
- Are men who elect to receive VMMC also more likely than an average member of the population to receive antiretroviral therapy if they acquire HIV?
- 124
A chart of the cost-effectiveness by country is here:
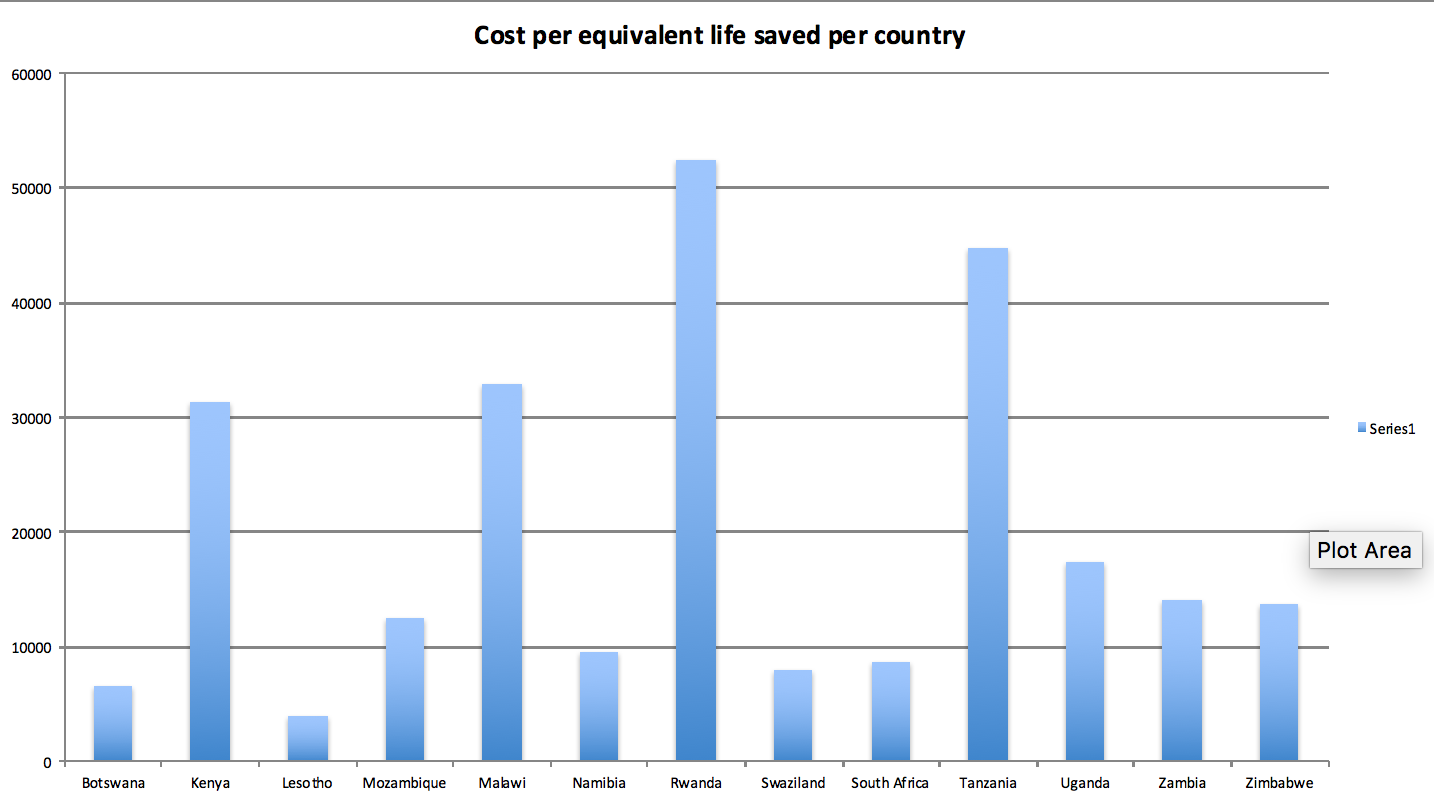
- 125
We expect giving opportunities to be situated in the 14 East and Southern African countries which the WHO has identified as priority countries for VMMC scale-up based on their high HIV prevalence rate and low circumcision rate: Bostwana, Ethiopia, Kenya, Lesotho, Malawi, Mozambique, Namibia, Rwanda, South Africa, Swaziland, Tanzania, Uganda, Zambia and Zimbabwe. See: "Table: annual numbers of male circumcisions in East and Southern Africa by country, 2008 – 2013, and progress towards goals", World Health Organization - Progress Brief: Voluntary Medical Male Circumcision for HIV Prevention in Priority Countries – 2014.
- 126
- We use the mean 2013 WHO estimate of the HIV prevalence rate among adults aged 15-49 in VMMC priority countries (as set out in the "extraction + calcs" sheet) sourced from World Health Organization - Data on Prevalence of HIV Among Adults Aged 15 to 49 - 2014. We did not vet these estimates.
- "There is currently insufficient evidence of individual or public health benefit to recommend male circumcision for HIV-positive men. Since persons with severe immunodeficiency may have increased complication rates following surgery, male circumcision in HIV-infected men should only be recommended when it is medically indicated … If male circumcision is requested by men with HIV infection following in-depth counselling on the known risks and benefits, it should not be withheld unless it is medically contraindicated", World Health Organization/UNAIDS' New Data on Male Circumcision and HIV Prevention - Policy and Programme Implications - 2007, Pg. 11.
- PEPFAR's practices are consistent with WHO Guidelines: "The limits of the protective benefits of VMMC should be explained to HIV-positive men and their partners, and if a client requests VMMC anyway (for reasons other than HIV prevention) and is healthy enough and is clinically fit (i.e., his CD4 count is above the treatment initiation threshold), VMMC should be made available to him", PEPFAR's Best Practices for Voluntary Medical Male Circumcision Site Operations - 2013, Pg. 28.
- We assume that all men who test HIV-positive will nevertheless choose to be circumcised, for reasons other than HIV prevention. We draw this information from the experience of the Centre for HIV and AIDS Prevention Studies (CHAPS), a South Africa-based VMMC charity: “CHAPS' experience is that men who test positive for HIV (and therefore do not derive significant medical benefits from circumcision) nevertheless choose to proceed with circumcision surgery. CHAPS conducts surgeries on HIV-positive men so long as the surgery will not jeopardize their health. The percentage of CHAPS' clients who test positive for HIV closely follows the average national prevalence rate for the relevant age group.” GiveWell's non-verbatim summary of a conversation with Dr. Dirk Taljaard, co-CEO of the Centre for HIV and AIDS Prevention Studies, March 22, 2016. We would expect to refine this premium to match information a VMMC charity provided.
- 127
Note that we subtract the incidence risk ratios found in Siegfried et al 2009 from 1 to get what we call the “risk reduction rate” above: "We combined the survival estimates for all three trials at 12 months and also at 21 or 24 months in a meta-analysis using available case analyses using the random effects model. The resultant incidence risk ratio (IRR) was 0.50 at 12 months with a 95% confidence interval (CI) of 0.34 to 0.72; and 0.46 at 21 or 24 months (95% CI: 0.34 to 0.62)", Siegfried et al 2009, Pg. 2.
- 128
- For example, assuming a risk reduction rate of roughly 0.52 may be an overestimate because:
- The trials were stopped early, which may have led the treatment effect to be overestimated.
- The effect is somewhat imprecisely estimated, so taking a point estimate from the center of the confidence interval may lead to a slight overestimate by chance.
- On the other hand, a risk reduction rate of roughly 0.52 may be an underestimate for reasons including:
- There is some evidence from the trial follow-ups that VMMC's protective effect may slightly increase over time.
- "The efficacy of MMC was sustained at 58% at 72 months", Mehta et al 2013, Pg. 2899.
- "Following trial closure we offered MC to uncircumcised control arm participants maintained surveillance for up to 4.79 years … In control arm participants, post-trial HIV incidence was 0.54/100 py in circumcised and 1.71/100 py in uncircumcised control arm men (adjusted effectiveness 67% (95%CI 38-83%)", Gray et al 2012.
- If there were positive spillovers from the treatment to the control group during the RCTs (i.e., if the lower incidence rate in the treatment group also led to lower incidence in the control group), then the positive effect of VMMC would be underestimated.
- The effect is somewhat imprecisely estimated, so taking a point estimate from the center of the confidence interval may lead to a slight underestimate by chance.
- There is some evidence from the trial follow-ups that VMMC's protective effect may slightly increase over time.
- We are generally unsure whether to expect the relative risk reduction from VMMC to increase or decrease over time. Considerations include:
- Assuming that changes in the relative risk reduction of circumcision over time are primarily a function of sexual behavior, one might expect that the relative risk reduction would fall as one gets older (if older people engage in no risky sexual behavior, then the relative risk reduction may fall to 0 as absolute risk falls to 0; though, it is unclear how relative risk would be affected even at very low levels of risky sexual behavior). However, for as long as sexual behavior is similar to what was observed during the trial period, one might expect a similar relative risk reduction to persist as was seen during the trial.
- If the counseling provided during the RCTs played a role in reducing the relative risk of acquiring HIV, then one might expect the effects of counseling (and hence the relative risk reduction) to decrease over time.
- Since circumcision is irreversible, we do not see reason to expect the effect of the surgery itself to fade over time.
- For example, assuming a risk reduction rate of roughly 0.52 may be an overestimate because:
- 129
For calculations, see our cost-effectiveness analysis of VMMC, "VMMC extraction + calcs" sheet, Section 1, “Compound annual decrease” row. Data is sourced from: UNAIDS – AIDSinfo indicators – 2014.
- 130
We estimate the number of persons to remove per year for non-HIV related mortality as 60.8 per 10,000. We use the Global Burden of Disease study's 2013 estimate of the all-cause mortality rate for males aged 15-49 less its estimate of the HIV related mortality rate in Namibia, the priority country with the HIV prevalence rate closest to the mean. We have not vetted the relevant estimates. See the "HIV Modelling" sheet of our cost-effectiveness analysis for calculations. Data is sourced from GBD Compare - 2013.
- 131
Other assumptions include, but are not limited to:
- We do not account for the fact that men may typically experience different HIV incidence rates as they age.
- We do not explicitly model at what age we expect men to be circumcised.
- 132
This multiplier is consistent with that employed in Kahn, Marseille and Auvert 2006. At Pg. 2353, the authors explain: "The epidemic multiplier was used to portray the effect of three factors that cause infections prevented to deviate from the simple product of HIA [HIV infections avoided] in the first year and the number of years projected. The first factor is that HIV infections prevented in men with circumcisions will lead to HIA in female partners in the community. … A second factor that would enhance the benefits of MC is that, when a substantial number of men are circumcised and there is an indirect reduction in infections in women (as above), the lower HIV prevalence among women will further decrease risk to men. … Finally, the lower HIV prevalence in women resulting from the first infections averted by MC reduces the later incidence rate in susceptible men and hence the benefits of subsequent MC. … In order to estimate the combined effect of these three factors, and to conduct sensitivity analyses, we adapted a simple dynamic epidemic modeling approach reported previously (described in Protocol S1). Overall, the 'epidemic multiplier' representing all three factors was estimated as 1.53; we use 1.5 (range 1.0–2.0)."
- 133
See this section of the report.
- 134
See spreadsheet, Sheet "CEA", Cell C19.
- 135
Note that our model effectively assumes that for every one male who is circumcised, there will be one female who has a 30% relative risk reduction of acquiring high-risk HPV. See spreadsheet, Sheet “CEA”, Cell C28.
- 136
See our VMMC CEA, Sheet “VMMC CEA”, Row “Discount to account for the fact that some of the population are not monogamous.”
- 137
Table 1, Arbyn et al 2011, Pg. 2676.
- 138
"In those countries for which reliable temporal data are available, incidence rates [of HPV] appear to be consistently declining by approximately 2% per annum", Forman et al 2012, Abstract.
- 139
We formed this impression through speaking with experts, reviewing resources on the websites of the Center for Disease Control and Priorities, the National Health Service, and the National Institutes of Health, as well as searching on Google Scholar.
- 140
"People with weak immune systems may be less able to fight off HPV and more likely to develop health problems from it, this includes people with HIV/AIDS", Centers for Disease Control and Prevention – Genital HPV Infection Factsheet – 2014, Pg. 1.
- 141
In particular, we discount by ~20% for each of three factors: external validity, replicability, and uncertainty in how we have modeled the relationship between HPV and cervical cancer. See spreadsheet, Sheet “CEA”, Cell C38.
- 142
For example, we have seen: Kahn, Marseille and Auvert 2006, USAID Health Policy Initiative - Decision-Makers’ Program Planning Tool - Cost and Impact of Male Circumcision - 2010, and Njeuhmeli et al 2011.
- 143
See:
- "The cost is $181 (80% CI $117–$306) per HIA [HIV infection averted]", Kahn, Marseille and Auvert 2006, Pg. 2349.
- "2008-15 : $1,676" and "2008-25 : $414", USAID Health Policy Initiative - Decision-Makers’ Program Planning Tool - Cost and Impact of Male Circumcision - 2010, Sheet "Cost per Infection Averted", cells N26-7 and T26-7.
- "Combining the cost data with the number of HIV infections averted, we obtain the discounted cost per HIV infection averted. The cost per HIV infection averted for the period 2011–2025 ranges from US$369 in Zimbabwe, where adult HIV prevalence is 17.9%, to US$4,096 in Rwanda, where adult HIV prevalence is lower than in any of the other 13 countries studied (3.1%). The overall cost per HIV infection averted for 2011–2025 for all 13 countries is US$809", Njeuhmeli et al 2011, Pg. 7.
- 144
See, generally, "South African HIV-positive adults can have a near-normal life expectancy, provided that they start ART before their CD4 count drops below 200 cells/µl", Johnson et al 2013, Pg. 1; and "Mortality of HIV-infected patients treated with combination ART in sub-Saharan Africa continues to be higher than in the general population, but for some patients excess mortality is moderate and reaches that of the general population in the second year of ART. Much of the excess mortality might be prevented by timely initiation of ART", Brinkhof et al 2009, Pg. 1.
- 145
- See, generally, "The median time from seroconversion to death was 9.0 years (N = 240) and 6.2 years to a CD4 cell count less than 200 cells/µl or WHO stage 4 (N = 229)", Van der Paal et al 2007, Pg. S21; "The median time from seroconversion to AIDS was 9.4 years and from AIDS to death was 9.2 months" Morgan et al 2002, Pg. 597; "Median estimated survival [in developing countries] from HIV seroconversion appears to be 8–9 years for individuals infected at 20–29 years, and is considerably shorter for older ages", Porter and Zaba 2004, Pg. S9.
- These results are congruent with the results of a significant pooled cohort study of HIV incident cases from the developed world, which found that mortality at 10 years following HIV infection without treatment was 0.44: "The cumulative incidence of all cause mortality was 0.10 at 5 years and 0.44 at 10 years following seroconversion in the pre-HAART era", CASCADE Collaboration 2006, Pg. 743.
- 146
"The updated findings presented here cover the time from 1990 until the end of 2003 (after which HAART was introduced in this cohort)" and "Eligible individuals at least 15 years of age with documented HIV seroconversion were recruited from a general population cohort in rural Uganda … We recruited 240 HIV incident cases," Van der Paal et al 2007, Pg. S21-2.
- 147
Figure 3(b), Van der Paal et al 2007, Pg. S26; see also "The median time from seroconversion to death was 9.0 years (N = 240) and 6.2 years to a CD4 cell count less than 200 cells/µl or WHO stage 4 (N = 229)", Van der Paal et al 2007, Pg. S21.
- 148
- On untreated persons: "79% of eligible persons in Western and Central Africa" and "59% of eligible persons in Eastern and Southern Africa are not receiving [anti-retroviral treatment]", UNAIDS - Access to Anti-Retroviral Therapy in Africa - 2015, Pg. 4. We have not vetted these estimates. See our cost-effectiveness analysis, "VMMC extraction + calcs" sheet, Section 5, “Weighted average” row, for the weighted average of these estimates.
- On eligibility criteria: "WHO 2013 consolidated guidelines on use of antiretroviral drugs, recommend: Antiretroviral therapy (ART) should be initiated for all adult patients with a CD4 count equal or less than 500 cells per mm3", UNAIDS - Access to Anti-Retroviral Therapy in Africa - 2015, Pg. 2.
- On CD4 cells and their importance: "A very low CD4 count (less than 200 cells/mm3) is one of the ways to determine whether a person living with HIV has progressed to stage 3 infection (AIDS)", Aids.gov - CD4 Count – 2015.
- 149
See Salomon et al 2015, Pg. e717.
- 150
"Left without treatment, the majority of people infected with HIV will develop signs of HIV-related illness within 5–10 years, although this can be shorter. The time between acquiring HIV and an AIDS diagnosis is usually between 10–15 years, but sometimes longer", World Health Organization – HIV/AIDS – 2015.
- 151
"The median time from seroconversion to death was 9.0 years (N = 240) and 6.2 years to a CD4 cell count less than 200 cells/µl or WHO stage 4 (N = 229)", Van der Paal et al 2007, S21; "The median time from seroconversion to AIDS was 9.4 years and from AIDS to death was 9.2 months", Morgan et al 2002, Pg. 597.
- 152
"Many of the severe symptoms and illnesses of HIV disease come from the opportunistic infections that occur because your body's immune system has been damaged", Aids.gov – Symptoms of HIV – 2015.
- 153
"If you have HIV and you are not on ART, eventually the virus will weaken your body's immune system and you will progress to AIDS (acquired immunodeficiency syndrome), the late stage of HIV infection. Symptoms can include: rapid weight loss; recurring fever or profuse night sweats; extreme and unexplained tiredness; prolonged swelling of the lymph glands in the armpits, groin, or neck; diarrhea that lasts for more than a week; sores of the mouth, anus, or genitals; pneumonia; red, brown, pink, or purplish blotches on or under the skin or inside the mouth, nose, or eyelids; and memory loss, depression, and other neurologic disorders", Aids.gov – Symptoms of HIV – 2015.
- 154
Sub-headings on Pg. 30-2, Nyblade and Ogden 2005.
- 155
World Health Organization Regional Office for Africa - Cervical Cancer: Issues and Challenges – 2015.
- 156
Table 1, Arbyn et al 2011, Pg. 2676, Estimate given for developing countries, calculation made by dividing the cumulative rate of dying from cervical cancer before the age of 75 years (1.1%) by the cumulative rate of developing cervical cancer (1.9%) before the age of 75 years.
- 157
Table 1, Arbyn et al 2011, Pg. 2676, Weighted average of the estimates given for Eastern, Middle and Southern Africa. For calculations, see our cost-effectiveness analysis, “VMMC extraction + calcs” sheet, Section 4, “Mortality rate” row.
- 158
"Cervical cancer may spread (metastasize) within the pelvis, to the lymph nodes or elsewhere in the body. Signs of advanced cervical cancer include: weight loss; fatigue; back pain; leg pain or swelling; leakage of urine or feces from the vagina; and bone fractures", Cancer Treatment Centers of America – Cervical Cancer Symptoms - 2015.
- 159
World Health Organization Regional Office for Africa - Cervical Cancer: Issues and Challenges – 2015.
- 160
"Age-specific analyses clearly indicate that cervical cancer primarily affects young adult women who are actively involved in their careers or caring for their families", Arbyn et al 2011, Pg. 2681-2.
- 161
"In five countries where voluntary medical male circumcision is stated to be a priority (Lesotho, Malawi, Namibia, Rwanda and Zimbabwe), coverage of voluntary medical male circumcision for adults is less than 10%", UNAIDS - Global Report - 2013, Pg. 20.
- 162
See "Table: annual numbers of male circumcisions in East and Southern Africa by country, 2008 – 2013, and progress towards goals", World Health Organization - Progress Brief: Voluntary Medical Male Circumcision for HIV Prevention in Priority Countries – 2014; and the following studies reporting relevant qualitative evidence in relation to demand for VMMC:
- Westercamp and Bailey 2007 conducted a review of studies of the acceptability of VMMC in Sub-Saharan Africa. Based on 13 studies from 9 countries, the median proportion of uncircumcised men willing to become circumcised was 65% (95% CI: 29-87%) (see Abstract, Pg. 341).
- Hatzold et al 2014 conducted a population-based survery of 1165 men aged 15-49 in Zimbabwe. The authors found that 11.3% reported being circumcised and 49% reported willingness to undergo VMMC (see Abstract, Pg. 1).
- 163
See, eg, "The U.S. Agency for International Development (USAID), with funds from the U.S. President's Emergency Plan for AIDS Relief (PEPFAR), has been at the forefront of introducing, launching and rolling out this effective HIV prevention method in several of the priority countries. … USAID works in close collaboration with the Bill & Melinda Gates Foundation; the Global Fund to Fight AIDS, Tuberculosis and Malaria; WHO; UNAIDS; UNICEF; the World Bank; U.S. Government agencies implementing the PEPFAR program (the Centers for Disease Control and Prevention, the Department of Defense, the National Institutes of Health and the Office of the U.S. Global AIDS Coordinator); and implementing partners", USAID – Accelerating the Scale-Up of VMMC - 2015.
- 164
"Voluntary medical male circumcision resource needs, Projected Available - $790 million, Gap - $710 million, Total: $1.5 billion. Additional investment needed by 2016 for 80% coverage in 14 priority countries", Clearinghouse on Male Circumcision for HIV Prevention – Update on Priority Countries – 2014, Pg. 2.
- 165
"…the US $8.75 group had significantly higher VMMC uptake than the control group (adjusted odds ratio [AOR] 4.3; 95% CI, 1.7-10.7), as did the US $15.00 group (AOR 6.2; 95% CI, 2.6-15.0). Effect sizes for the US $8.75 and US $15.00 groups did not differ significantly (P = .20)”, Thirumurthy et al 2014, Pg. 703.
- 166
"In June 2007 we searched the following electronic journal and trial databases: MEDLINE, EMBASE, and CENTRAL. We also searched the electronic conference databases NLM Gateway and AIDSearch and the trials registers ClinicalTrials.gov and Current Controlled Trials. We contacted researchers and relevant organizations and checked reference lists of all included studies", Siegfried et al 2009, Pg. 1.
